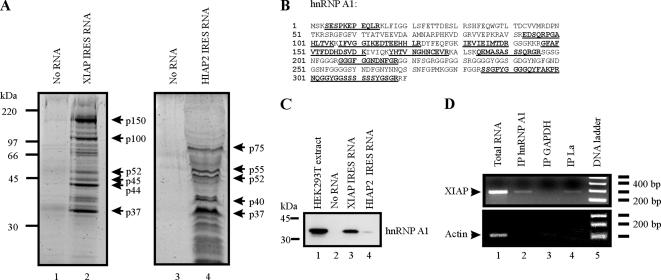
Figure 1.
hnRNP A1 interacts with the XIAP IRES. (A) RNA-affinity chromatography isolation of XIAP IRES-binding proteins. Precleared protein extracts from HEK293T cells were incubated with either agarose beads coated with XIAP IRES RNA, agarose beads coated with HIAP2 IRES RNA, or agarose beads alone. After protein binding, beads were washed extensively and pelleted, and proteins were eluted by boiling and resolved by SDS-PAGE. Proteins were visualized with Sypro Ruby stain, and the indicated protein species were excised from the gel and subjected to mass spectrometry analysis. (B) The p37 protein is hnRNP A1. Underlined peptide sequences were identified by mass spectrometry analysis of the p37 protein species isolated by RNA-affinity chromatography using XIAP IRES RNA as an affinity matrix. (C) hnRNP A1 associates with XIAP IRES RNA in vitro. XIAP IRES RNA and HIAP2 IRES RNA were used in RNA-affinity chromatography as described in A; isolated proteins were separated by SDS-PAGE, transferred to PVDF membrane, and probed with anti-hnRNP A1 antibody. (D) hnRNP A1 associates with XIAP mRNA in vivo. RNA–protein complexes were cross-linked with formaldehyde, isolated from cells, and immunoprecipitated using antibodies against hnRNP A1, La, and GAPDH. After immunoprecipitation, RNA–protein cross-links were reversed, and the RNA was isolated and used in an RT-PCR reaction with XIAP- and actin-specific oligonucleotide primers.