Figure 3.
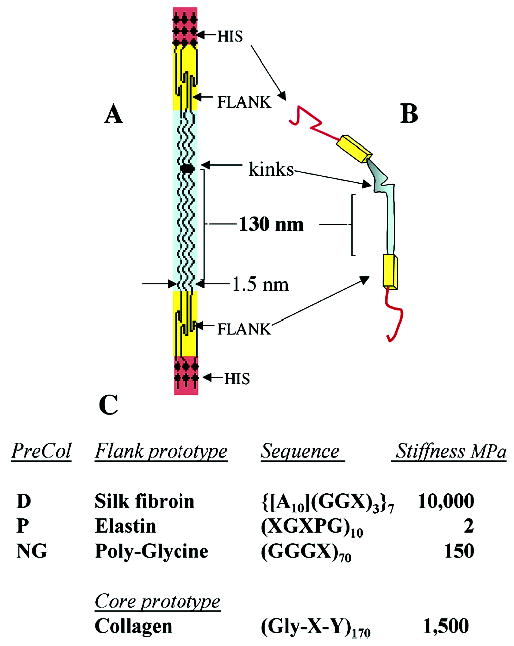
Proposed structure of the preCOLs making up the bulk of each mussel byssal thread. The block domain structure in 2D of a trimer is shown in panel A; the bent-core analogue of a trimer is shown in panel B; amino to carboxy terminal orientation is top-to-bottom. Summary of sequence features of each flank type and estimated stiffness constants (panel C) are as described in text. X represents any amino acid except glycine and alanine. Stiffness represents the initial modulus determined for fully hydrated biopolymers including the alanine-rich fibroin of Anaphe silk (14).
