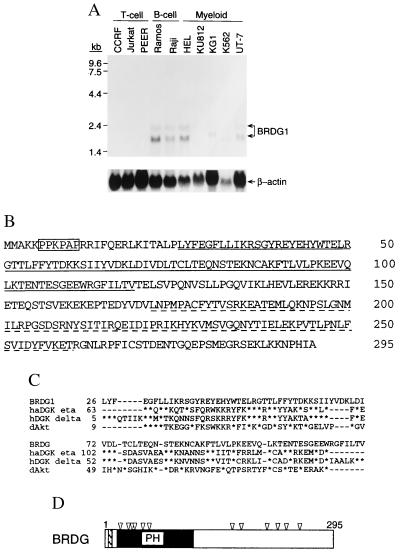
Figure 1.
(A) Northern blot analysis of total RNA (20 μg per lane) from CCRF-CEM (CCRF), Jurkat, PEER, Ramos, Raji, HEL, KU812, KG1, K562, and UT-7 cells with the 32P-labeled BRDG1 cDNA (top row) or β-actin cDNA (bottom row). The positions of molecular size standards (in kilobases) are shown on the left and the hybridizing transcripts are shown on the right. (B) The deduced amino acid sequence of human BRDG1. The sequence is shown in single-letter notation, with the PH domain and the putative SH2 domain indicated by solid and broken underlines, respectively. The proline-rich motif is boxed. Residue numbers are on the right. (C) Comparison of the amino acid sequence of BRDG1 with those of golden hamster DGK-η (haDGK eta; GenBank accession no. Q64398), human DGK-δ (hDGK delta; accession no. D73409), and the D. discoideum Akt homolog (dAkt; accession no. P54644). Residues identical with those of BRDG1 are shown as asterisks; dashes represent gaps introduced to optimize alignment. Residue numbers are shown on the left. (D) Overall structure of BRDG1. The proline-rich motif and the PH domain are shown as hatched and solid boxes, respectively. The positions of tyrosine residues are indicated by arrowheads.