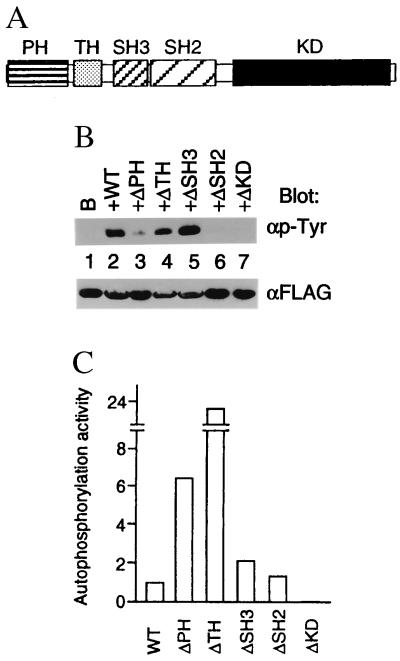
Figure 3.
(A) Organization of the Tec protein into PH, TH, SH3, SH2, and kinase (KD) domains. (B) Effect of various deletion mutants of Tec on BRDG1 phosphorylation in 293 cells. Recombinant BRDG1 was immunoprecipitated with anti-FLAG antibody from 293 cells expressing BRDG1 (B) either alone or together with wild-type Tec (WT) or Tec mutants lacking (Δ) the indicated domains. The resulting precipitates were then subjected to immunoblot analysis with antibodies to phosphotyrosine (αp-Tyr) or FLAG (αFLAG). (C) Autophosphorylation activity of various Tec mutants. Wild-type Tec and the various Tec mutants used in B were immunoprecipitated from transfected 293 cells and subjected to immunoblot analysis with either anti-phosphotyrosine antibody or anti-Tec antibody. Autophosphorylation activity of Tec and its mutants were shown in arbitrary units as tyrosine phosphorylation intensity per protein amount, both measured by a densitometer.