Fig.7.
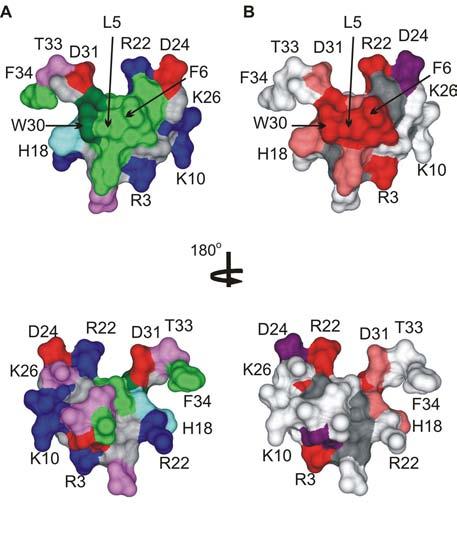
The active surface of SGTx.
(A) Surface rendering of the SGTx structure illustrating the amphipathic nature of the toxin. Residue coloring as follows: blue, basic; red, acidic; violet, Ser/Thr; green, hydrophobic (Trp is dark green). (B) Surface rendering of SGTx colored according to mutagenesis results. Backbone atoms and unstudied residues (L19, Y27 and 6 Cys) are colored dark gray. Mutants that do not significantly alter Kd (|ΔΔG| < 1 kcal mol−1) are colored light gray. Mutants that display weaker binding affinity are colored pink if |ΔΔG| = 1-1.5 kcal mol−1 and red if |ΔΔG| > 1.5 kcal mol−1. Two mutants displaying stronger binding affinity are colored purple. See (Wang et al., 2004) for details. Bottom images were obtained by rotating around the axis shown. Images were created us DSViewer Pro and Protein Data Bank accession IDs 1LA4 for SGTx (Lee et al., 2004).
