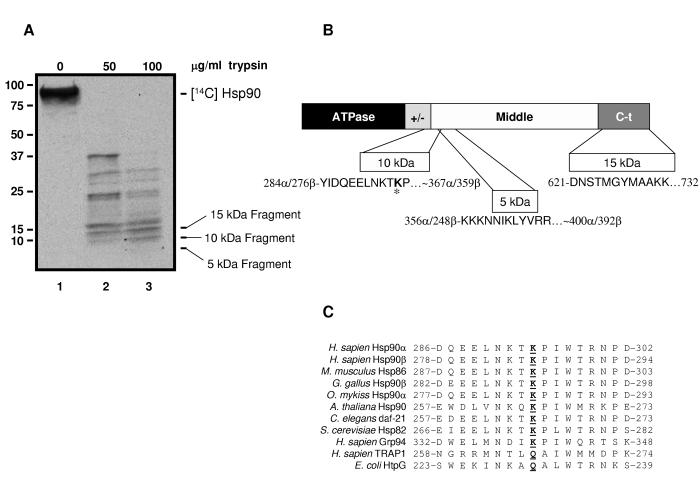
Figure 1.
Hsp90 is Acetylated in More Than One Domain
(A) SkBr3 cells were labeled with [14C]acetate. Hsp90 was immunoprecipitated from cell lysate, digested with 50 (lane 2) or 100 μg/ml trypsin (lane 3), and fragments were separated by SDS-PAGE and transferred to PVDF membrane. Acetylated bands were detected by autoradiogram. The 3 indicated bands were sequenced.
(B) Diagram of Hsp90 domain architecture showing location and N-terminal sequence of the bands indicated in (A). (ATPase = ATPase domain, +/-= charged linker region, Middle = middle domain, C-t = carboxy-terminal domain; the human Hsp90αsequence was used to determine amino acid numbers). K294 is identified by bolding and an asterisk.
(C) Diagram of K294 relative to Hsp90 domain architecture (domains are as indicated in Fig. 1B). Below is an alignment of the sequence including and around K294 from different organisms and isoforms of Hsp90. K294 or corresponding residues in other organisms/isoforms is underlined and in bold.