Abstract
The ability of a cell to sense and respond to DNA damage is essential for genome stability. An important aspect of the response is arrest of the cell cycle, presumably to allow time for repair. Ataxia telangiectasia mutated (ATM) and ATR are essential for such cell-cycle control, but some observations suggest that they also play a direct role in DNA repair. The Drosophila ortholog of ATR, MEI-41, mediates the DNA damage-dependent G2-M checkpoint. We examined the role of MEI-41 in repair of double-strand breaks (DSBs) induced by P-element excision. We found that mei-41 mutants are defective in completing the later steps of homologous recombination repair, but have no defects in end-joining repair. We hypothesized that these repair defects are the result of loss of checkpoint control. To test this, we genetically reduced mitotic cyclin levels and also examined repair in grp (DmChk1) and lok (DmChk2) mutants. Our results suggest that a significant component of the repair defects is due to loss of MEI-41-dependent cell cycle regulation. However, this does not account for all of the defects we observed. We propose a novel role for MEI-41 in DSB repair, independent of the Chk1/Chk2-mediated checkpoint response.
CELL-CYCLE regulation is an important response to DNA damage. This regulation couples repair with cell-cycle progression to prevent genomic instability following DNA damage. In the DNA damage checkpoint pathway, sensors recognize DNA damage and then stimulate a variety of responses, including phosphorylation of transducers of the checkpoint pathway. These transducers then activate or inactivate effectors that directly inhibit cell-cycle progression, resulting in arrest of the cell cycle to allow time to repair the damage (reviewed in Sancar et al. 2004).
Ataxia telangiectasia mutated (ATM) and ATR are important mediators of DNA damage checkpoints. Both regulate DNA damage-dependent cell-cycle checkpoints at the G1-to-S transition, within S phase, and at the G2-to-M transition (reviewed in Shiloh 2003; Sancar et al. 2004). In mammals, ATM responds primarily to double-strand breaks (DSBs) and is therefore activated by ionizing radiation (IR) (Canman et al. 1998). In contrast, ATR responds primarily to the presence of ssDNA and is therefore activated by hyperoxia, DNA polymerase inhibitors, and ultraviolet (UV) radiation (Cliby et al. 1998; Wright et al. 1998; Unsal-Kacmaz et al. 2002; Das and Dashnamoorthy 2004). Recent findings, however, suggest that the roles of these checkpoints may be more complex than originally proposed. In mammals, there is evidence that ATM and ATR may regulate each other. For example, ATR may be activated by ssDNA that is generated during ATM-dependent repair of DSBs (Wang et al. 2003). Also, ATR is required to maintain the checkpoint initiated by ATM (Brown and Baltimore 2003).
Although there are Drosophila orthologs for both ATM and ATR, the DNA damage-dependent checkpoint functions reside primarily in the latter, which is encoded by the mei-41 gene. The MEI-41 protein is required to prevent entry into mitosis before completion of replication and for checkpoints induced by DSBs during all phases of the cell cycle (Hari et al. 1995; Sibon et al. 1999; Brodsky et al. 2000; Garner et al. 2001; Jaklevic and Su 2004; Bi et al. 2005a). As a consequence, mei-41 mutants are hypersensitive to agents that inhibit or block replication, such as hydroxyurea, alkylating chemicals, and ultraviolet radiation, and to agents that generate DSBs, such as IR (Boyd et al. 1976; Sibon et al. 1999). In contrast, the primary roles of the Drosophila ortholog of ATM (encoded by tefu) are in telomere stabilization and regulation of p53-dependent apoptosis; null mutations in tefu are lethal, possibly due to chromosome breakage resulting from telomere fusions (Bi et al. 2004, 2005b; Silva et al. 2004; Song et al. 2004). Temperature-sensitive tefu mutants are hypersensitive to IR, but they have a fully functional checkpoint at high doses of IR (Silva et al. 2004). Thus, although MEI-41 has more sequence similarity to ATR, it appears to function as the major component of replication and DNA damage checkpoint pathways similar to both mammalian ATR and ATM. These checkpoint functions appear to be mediated entirely by orthologs of Chk1 and Chk2, which are encoded by the grp and lok genes, respectively (Xu et al. 2001; Brodsky et al. 2004; Jaklevic and Su 2004; de Vries et al. 2005).
Most studies of ATM and ATR have focused on the checkpoint functions of these kinases. However, it has been suggested that human ATM has a role in DNA repair in addition to its role in cell-cycle regulation (reviewed in Jeggo et al. 1998; Lobrich and Jeggo 2005; Jeggo and Lobrich 2006; O'Driscoll and Jeggo 2006). Numerous studies addressing the possible role of ATM and ATR in DSB repair followed the observation that cells from ataxia telangiectasia (A-T) patients, which are defective for ATM, are radiosensitive (Taylor et al. 1975). Several studies found that ATM is involved in Artemis-dependent nonhomologous end-joining (NHEJ) repair of DSBs (Kuhne et al. 2004; Riballo et al. 2004; Jeggo and Lobrich 2005). ATM has also been suggested to facilitate HR repair in chicken DT40 cells, on the basis of synergistic sensitivity to IR when both ATM and NHEJ genes are deleted (Morrison et al. 2000). ATM- and ATR-dependent phosphorylation of proteins that facilitate the assembly of repair complexes on damaged DNA, such as histone H2AX, also suggests a role for these kinases in regulation of DSB repair (reviewed in Paull et al. 2000; Abraham 2001; Ward and Chen 2001; Bassing et al. 2002; Celeste et al. 2002). Similarly, several lines of evidence implicate the Saccharomyces cerevisiae ortholog of ATR, Mec1, in DSB repair. For example, Mec1 is required for localization of components of the Sir complex, which has been implicated in NHEJ, after DSB induction (Mills et al. 1999) and for phosphorylation of histone H2A, which is required for efficient NHEJ. Together, these studies support the existence of an important interplay between cell-cycle checkpoint regulation and DNA repair. Nonetheless, the roles of ATM and ATR seem to vary in different studies, and the consequences of removal of these kinases on repair processes are not fully understood.
Drosophila MEI-41 has also been proposed to have a checkpoint-independent role in promoting survival after IR. Jaklevic and Su (2004) found that mei-41 mutants are killed by doses of IR that are not lethal to grp mutants, although both are defective in the G2-M checkpoint induced by IR. Similarly, Oikemus et al. (2006) found that both spontaneous and IR-induced chromosome breaks were increased in mei-41 mutants but not in grp lok double mutants, suggesting that MEI-41 has a role in preventing chromosome breaks that is independent of GRP and LOK.
To better understand specific roles of MEI-41 in DNA repair, we assayed DSB repair in mei-41 mutants. Our results indicate that the ability to complete the later steps of repair by homologous recombination (HR) in the absence of MEI-41 is decreased, frequently resulting in cell or organismal lethality. We did not detect an effect of loss of MEI-41 on repair through NHEJ. Our data suggest that DSB repair defects in mei-41 mutants are due in part to the role of MEI-41 in eliciting the G2-M DNA damage checkpoint, but that loss of the GRP/LOK-mediated checkpoint does not account for all of the defects observed. This reveals a novel role for MEI-41 in HR repair that is independent of the known function in cell-cycle regulation.
MATERIALS AND METHODS
Drosophila stocks and genetics:
Flies were maintained on standard medium at 25°. The P{wa} transgene used in this study is described by Kurkulos et al. (1994) and Adams et al. (2003). The mei-41 mutant males were hemizygous for mei-4129D (Laurencon et al. 2003). The spn-A mutants were compound heterozygotes of spn-A057 and spn-A093A (Staeva-Vieira et al. 2003). The cyclin alleles used were CycAC8LR1 (Sigrist and Lehner 1997) and CycB2 (Jacobs et al. 1998).
The grp mutants used were compound heterozygotes of grp209 and grpZ5170. grpZ5170 was obtained by screening a collection of nonlethal EMS mutants on the second chromosome (Koundakjian et al. 2004) for maternal-effect lethality and failure to complement grp1 (Fogarty et al. 1997). We sequenced the region encoding GRP in this mutant, revealing a C-to-T transition that changes a conserved proline in the kinase domain (residue 189) to leucine. The grp209 allele was generated by excision of P{EP}587, which is inserted 551 bp upstream of the initiator ATG site. Excision alleles were screened for failure to complement the female-sterile phenotype caused by grp1. A deletion in grp209 includes the first two coding exons (see below). Both alleles were confirmed to be genetic nulls by the observation that each was completely defective in the G2-M checkpoint after IR and failed to complement the maternal-effect embryonic lethality reported for other alleles. lok30 is a deletion of the 5′-UTR and first two coding exons, generated through excision of P{EPgy2}EY15840. This allele behaves like published null alleles in that mutants are moderately defective in the G2-M checkpoint after exposure to IR.
Viability experiments:
Male viability was determined by the ratio of P-element-containing males of the indicated genotype with transposase compared to the same genotype without transposase (equaling percentage of expected). Experiments with mei-41, spn-A, CycA, and/or CycB heterozygotes used the H{w+, Δ2-3}Hop2.1 transposase source, located on a CyO balancer chromosome. All flies carried a P{wa} inserted on the X chromosome. Experiments with grp and lok, which are on chromosome 2, used the P{ry+, Δ2-3}(99B) transposase source on chromosome 3. Although this source results in greater mosaicism in the eye, it produces fewer excisions in the male germline, but this does not affect repair outcomes (McVey et al. 2004a). Using the third chromosome transposase source, viability in mei-41 mutants was unaffected in the presence of the single P{wa} element, presumably due to lower activity of this source in some essential tissue; decreased transposase activity has been shown to decrease viability defects in other mutants (Engels et al. 1987). To compensate for the lesser transposase activity, we scored viability defects in a background with increased numbers of P elements, which would result in more cells experiencing at least one excision. We used the snw allele, which contains two P elements in the 5′-UTR of sn (Banga et al. 1991) and the original P{wa} element as three sources of excision. Wild-type, mei-41, and CycA and/or CycB heterozygotes were also tested in this background for comparison.
P{wa} assay:
Crosses for the P{wa} assay are described by Adams et al. (2003) and McVey et al. (2004a). Briefly, single males of each genotype containing P{wa} and the P{ry+, Δ2-3}(99B) transposase source on chromosome 3 were crossed to four y w P{wa} virgin females, and female progeny without transposase were scored for eye color. To compensate for changes in P{wa} excision rates (see results for details), we repeated each experiment two to four times for each genotype. Rates of completed synthesis-dependent strand annealing (SDSA) were determined by the number of completed SDSA events (red-eyed progeny) out of total repair events (red- and yellow-eyed progeny). Weighted averages were applied to compensate for changes in sample size between experiments. Standard deviation was determined by the weighted averages of the percentage of progeny showing completed SDSA. Aberrant repair products recovered in yellow-eyed females were analyzed in white-eyed sons of these females. To ensure that independent events were analyzed, only one such female was used from each cross vial.
Molecular analysis of aberrant repair:
Repair synthesis tract lengths were determined as described in Adams et al. (2003). Genomic DNA was prepared from single male flies containing the aberrant repair product derived from experiments using the H{w+, Δ2-3}Hop2.1 transposase source, located on a CyO balancer chromosome. PCR reactions contained 10 mm Tris-HCl pH 9.0, 50 mm KCl, 2.5 mm MgCl2, 0.1% Triton X-100, 1.25 μm of each primer, 250 μm each dNTP, 2 μl of the genomic DNA prep, and Taq DNA in a 20-μl volume. PCR products were analyzed by agarose gel electrophoresis followed by ethidium bromide staining. Positive and negative controls were included in each set of reactions.
Single-strand annealing assay:
Males that were wild type or mutant for mei-41, grp, and/or lok and carried the single-strand annealing (SSA) transgene (Rong and Golic 2000) and heat-shock-inducible I-SceI enzyme were heat-shocked at 38° for 1 hr to induce DSBs in germline and somatic tissues. Individual males were then crossed to wild-type females, and progeny were scored for SSA events on the basis of red (no SSA) or white (repair through SSA) eye color.
Sequencing of mutations:
The grp coding region was sequenced for changes in grpZ5170. Individual flies homozygous for the mutation were homogenized and PCR was performed using gene-specific primers. PCR products were isolated using gel electrophoresis, purified, and sequenced directly. The mutation was confirmed by sequencing the opposite strand. The genomic loci of grp209 and lok30 alleles were similarly sequenced to identify deletion breakpoints of the excision event.
Checkpoint assay:
We employed a well-established assay to determine defects in DNA damage checkpoints in mitotically dividing cells in imaginal discs after IR (Xu et al. 2001; Brodsky et al. 2004; Jaklevic and Su 2004; Bi et al. 2005a). Briefly, third-instar larvae were unirradiated or irradiated with either 500 or 4000 rad of γ-rays using a Gammator 50 irradiator and then kept at 25° for 1 hr. Imaginal discs were dissected in Ringer's solution and fixed in 4% formaldehyde and PBS with 0.1% Triton-X (PBT). Discs were washed and blocked in PBT with 5% bovine serum albumin (BSA). Discs were incubated with a 1:1000 dilution of rabbit anti-phospho-Histone H3 antibody (Upstate Biotechnology, Lake Placid, NY) in PBT overnight at 4°. Discs were incubated for 2 hr at room temperature with 1:1000 secondary goat anti-rabbit rhodamine-conjugated antibody (Molecular Probes, Eugene, OR), stained with 10 μg/ml DAPI in PBT, and mounted with Flouromount-G (Southern Biotechnology Associates). Discs were visualized using a TRIT-C filter of a Nikon Eclipse E800 fluorescent microscope.
RESULTS
mei-41 mutants are sensitive to transposase-induced DSBs due to defects in homologous recombination repair:
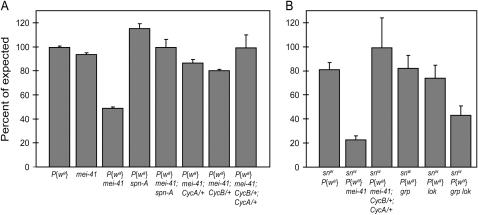
To investigate the role of MEI-41 in repairing DSBs, we employed a repair assay on the basis of excision of a P-transposable element (Adams et al. 2003). We generated males with an X chromosome insertion of P{wa} and P transposase, which catalyzes excision of P{wa} throughout development in both the germline and soma. Survival to adulthood of mei-41 males experiencing P{wa} excision during development is drastically reduced (Figure 1A), as in previous studies with other P-element insertions (Banga et al. 1991). Lethality was not observed in wild type or in mei-41 mutants that did not have a P{wa} insertion. Thus, MEI-41 plays a crucial role in responding to even a single DSB.
Figure 1.—
Lethality caused by transposase-induced DSBs. (A) Lethality using chromosome 2 transposase and a P{wa} element. (B) Lethality using chromosome 3 transposase and three P elements (P{wa} and two at the sn locus). DSBs occur in the somatic tissue and germline of the male progeny of the indicated zygotic genotype that have both the P{wa} element and transposase (A) or snw and P{wa} elements and transposase (B; see materials and methods for details). The percentage expected is determined by the number of males of the indicated genotype with transposase relative to brothers without transposase. Bars represent means; error bars indicate standard deviation from three independent experiments (wild-type, mei-41, and grp lok mutants) or range from two experiments (other genotypes).
We hypothesized that the decreased viability of mei-41 mutants is due to accumulated cell death caused by an inability to repair transposase-induced DSBs. Repair of these DSBs can occur through HR or NHEJ pathways. We inactivated HR with mutations in spn-A, which encodes the Drosophila ortholog of the strand invasion protein Rad51 (Staeva-Vieira et al. 2003; Figure 2b). Although spn-A mutants are incapable of repairing through HR and are extremely sensitive to ionizing radiation, these mutants can efficiently repair a single transposase-induced DSB through a noncanonical NHEJ pathway (McVey et al. 2004a; Figure 2c). Therefore, spn-A mutant males that have the X chromosome P{wa} insertion and transposase are fully viable (Figure 1A). Remarkably, mutations in spn-A completely suppressed the lethality of mei-41 mutant males associated with excision of the P{wa} element (Figure 1A). We propose that attempts to repair DSBs through HR in the absence of MEI-41 are frequently unsuccessful, resulting in an accumulation of cell death in some essential tissue and, consequently, organismal death. Analysis of individual repair events (see below) from mei-41; spn-A mutants supports this interpretation: all such repair events appear to result from end joining (data not shown), and the junctions are indistinguishable from those produced by spn-A single mutants (McVey et al. 2004a).
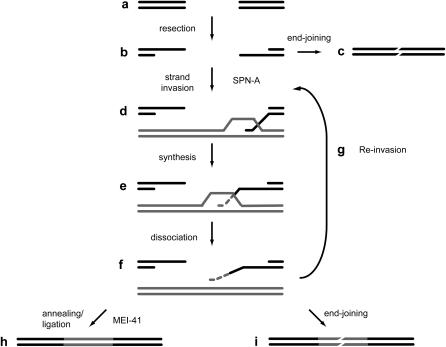
Figure 2.—
Model for repair of a gap through SDSA. Processing of a double-strand gap (a) begins with resection of the ends to leave 3′-ended single-stranded overhangs (b). The resected structure can then enter one of two pathways. The ends can join through a process that does not require extensive complementary ends (c) or one or both of these resected ends invade a homologous template (d) and prime repair synthesis (e). The nascent strand is dissociated, searching for a complementary single-stranded DNA to anneal to f. This structure resembles that of a resected product (b). Consequently, the nascent strand can reinvade a homologous sequence (g) or undergo end joining (i). Repair synthesis is not processive, so multiple rounds of synthesis, dissociation, and reinvasion (e–g) are required for repair across the gap (McVey et al. 2004a). Synthesis across the entire gap allows annealing of complementary single-stranded DNA, resulting in restoration of sequences lost when the gap was originally made (h). In the absence of SPN-A (DmRad51), repair occurs through an end-joining pathway (c). In the absence of MEI-41 (h), a majority of repair products that attempt the annealing/ligation steps are lost, resulting in a reduction of complete SDSA.
mei-41 mutants have reduced ability to complete the final steps of SDSA:
The viability defect described above demonstrates that attempts to repair DSBs through HR fail when MEI-41 is absent. To gain insight into the cause of the failure, we determined the molecular structures of repair events generated in mei-41 mutants. Germline DSB repair events were assayed by crossing surviving males with P{wa} and transposase to appropriate females and recovering repaired X chromosomes in the female progeny.
Most DSB repair in the Drosophila germline occurs through an HR pathway termed SDSA (described in Nassif and Engels 1993; Kurkulos et al. 1994; Adams et al. 2003; McVey et al. 2004a,b). SDSA begins with the generation of single-stranded DNA by resection of the 5′ ends of the DSB (Figure 2). One or both single-stranded ends can then invade a homologous template and prime repair DNA synthesis. After dissociation of the nascent strand from the template, SDSA is concluded through annealing of complementary single strands, trimming of any overhangs, filling of any gaps, and ligation.
Most female progeny from the surviving mutant males have apricot eyes; the majority of these are derived from cells in which the P element did not excise, but some result from complete restoration of the P{wa} element through SDSA, using the sister chromatid as a repair template (McVey et al. 2004a). Because we cannot distinguish between failure to excise and excision followed by restoration of the P{wa}, apricot-eyed progeny are uninformative. The other two classes of progeny, however, allow us to distinguish between complete SDSA (red eyes) and other repair mechanisms (yellow eyes) (Adams et al. 2003). Rates of P{wa} excision can vary, depending on transposase source and other factors, but this does not affect the ratio of the two types of repair events (McVey et al. 2004a; supplemental Table 1 at http://www.genetics.org/supplemental/). To control for differences in excision rate, repair outcomes are expressed as the percentage of detectable repair products (sum of red-eyed and yellow-eyed progeny) that have completed SDSA (red-eyed progeny; see supplemental data at http://www.genetics.org/supplemental/ for total numbers scored).
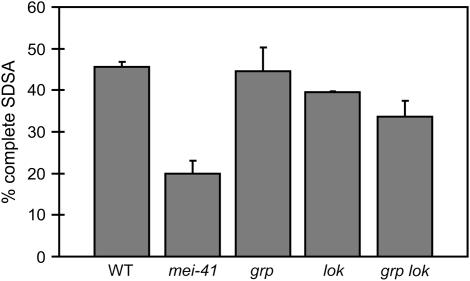
Of the repair events obtained in progeny of wild-type males, 46% were derived from completed SDSA (Figure 3). This class was significantly decreased in mei-41 mutants: Only 20% of repair events were completed SDSA, corresponding to a 57% reduction (P < 0.05). This reduction appears to be due to decreased recovery of SDSA repair products (progeny with red eyes), since there is neither a comparable decrease nor a corresponding increase in non-SDSA products (progeny with yellow eyes; supplemental Table 1 at http://www.genetics.org/supplemental/). This finding supports the proposal that in the absence of MEI-41, unsuccessful attempts to repair through SDSA result in cell death, rather than repair through another mechanism, such as end joining.
Figure 3.—
Percentage of SDSA repair events in checkpoint mutants. Bars represent average percentage of complete SDSA repair (red-eyed progeny) out of all repair products (red- and yellow-eyed progeny) from independent experiments of each indicated genotype. Error bars represent standard deviations from three or four independent experiments in wild type, mei-41, and grp lok mutants and ranges from two independent experiments in lok and grp single mutants. Statistical significance (P < 0.05) was determined from the weighted averages and weighted standard deviations of each genotype. Each independent experiment was an observation of a range of 63–387 individual repair events. See supplemental Table 1 at http://www.genetics.org/supplemental/ for total numbers scored.
To better understand the repair defect in mei-41 mutants, we determined the structures of repair events recovered as yellow-eyed progeny. Most of the yellow-eyed progeny of wild-type males result from initiation of SDSA followed by end joining rather than annealing and ligation (Adams et al. 2003). We found evidence for repair synthesis from one end of the break, utilizing a homologous template in 79 of 83 yellow-eyed progeny from wild-type males and 101 of 103 yellow-eyed progeny from mei-41 males. Because the P{wa} in this assay is on the male X chromosome, the only repair template available is the sister chromatid. Thus, excision and repair occur in G2 in both wild-type and mei-41 mutants.
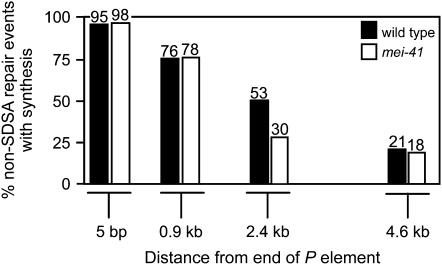
Relative to the sister chromatid, P{wa} excision generates a 14-kb gap. We previously reported evidence that repair DNA synthesis during SDSA is not highly processive and that repair of a large gap involves multiple rounds of strand invasion, synthesis, and dissociation (Figure 2; McVey et al. 2004a). Before each strand invasion event, the single-stranded ends may be joined through a noncanonical NHEJ pathway, resulting in an internally deleted P{wa} element. The amount of repair synthesis that occurs before end joining can be estimated by determining the lengths of synthesis tracts in repair events recovered in yellow-eyed progeny. Tract lengths were similar whether MEI-41 was present or not (Figure 4). We conclude that MEI-41 is not required for the early steps of SDSA, including initiation, strand invasion, repair synthesis, and dissociation (Figure 2, a–f). Likewise, MEI-41 is not required for completion of repair through NHEJ (Figure 2i).
Figure 4.—
Synthesis tract lengths in wild-type and mei-41 mutants. Repair synthesis from the right end of the DSB was analyzed for incomplete SDSA repair events (83 independent events from wild-type male flies and 103 from mei-41 mutants). Each bar represents the percentage of repair products with a synthesis tract at least as long as the indicated distance.
Our results suggest that the diminished ability of mei-41 mutants to repair DSBs through SDSA occurs during the final steps—annealing and ligation (Figure 2h). To confirm this conclusion, we assayed repair under conditions in which annealing and ligation are essential, but the need for strand invasion and repair synthesis is bypassed. When a DSB is made between directly repeated sequences, resection of 5′ ends exposes single-stranded sequences that can anneal without synthesis; this repair process is termed SSA (Ivanov et al. 1996). In the assay we employed (Rong and Golic 2000), >92% of the progeny of wild-type males resulted from repair by SSA (Table 1). In mei-41 mutants, 82% of the progeny resulted from SSA. Although the magnitude of the effect in this assay is lower than that in the SDSA assay, the decrease is highly significant (P < 0.0001).
TABLE 1.
Single-strand annealing (SSA) in checkpoint- defective mutants
| Genotype | n | % white (SSA) | % red (no SSA) |
|---|---|---|---|
| Wild type | 2574 | 92.0* | 8.0 |
| mei-41 | 2171 | 82.0** | 18.0 |
| lok | 769 | 95.3* | 4.7 |
| grp | 462 | 92.6* | 7.4 |
| grp lok | 2703 | 87.1*** | 12.9 |
P < 0.001 when compared to mei-41;
P < 0.001 when compared to wild type.
Taken as a whole, our results support the hypothesis that mei-41 mutants are unable to complete SDSA effectively, even when there is only a single DSB. In the absence of MEI-41, SDSA is initiated and repair synthesis proceeds as usual, likely involving multiple cycles of strand invasion, synthesis, and dissociation. Repair can then be completed by end joining without negative consequences. However, attempts to complete SDSA through annealing and ligation are frequently unsuccessful, resulting in cell death.
Lethality of mei-41 mutants is rescued by reducing mitotic cyclin levels:
We propose two hypotheses to explain the inability to complete SDSA in the absence of MEI-41. First, MEI-41 may facilitate the final steps of this pathway, perhaps by regulating enzymes important for these steps. Alternatively, or additionally, the loss of the G2-M DNA damage checkpoint in mei-41 mutants may result in entry into mitosis before repair by SDSA has been completed. To test this second hypothesis, we sought to delay entry into mitosis through other means and ask if these conditions bypass the requirement for MEI-41. Reducing the maternal contribution of the mitotic cyclins, cyclin A and cyclin B, slows early embryonic cell-cycle progression (Edgar et al. 1994) and can bypass the requirement for MEI-41 in regulating the midblastula transition during early embryonic development (Sibon et al. 1999). We found that reduction of the maternal contribution of CycA or of the zygotic contribution of either mitotic cyclin increased survival of mei-41 mutants carrying transposase and P{wa} (Figure 1, A and B, and data not shown). The combination of maternal and zygotic reduction in CycA plus zygotic reduction of CycB restored full viability.
We also determined the effects of cyclin reductions in a nonessential somatic tissue, the progenitors of the compound eye, as in Romeijn et al. (2005). In this assay, the eyes of males carrying P{wa} and transposase are scored for clones of red-pigmented cells, indicative of completed SDSA, and unpigmented (white) cells, indicative of repair through end joining (Adams et al. 2003). In wild-type flies, almost every eye has at least one red clone (supplemental Table 2 at http://www.genetics.org/supplemental/). In contrast, in mei-41 mutants, only 10% of eyes have red clones. In mei-41 mutants heterozygous for a CycA or CycB mutation, the frequency of red clones increased. This result, while not as precise as measurements of germline repair events, supports our conclusion that MEI-41-dependent regulation of cell-cycle progression contributes to the efficiency of HR repair in multiple tissue types.
Loss of the GRP/LOK-mediated G2-M DNA damage checkpoint accounts for only part of the decreased ability of mei-41 mutants to complete SDSA:
The finding that reducing the levels of mitotic cyclins can rescue lethality of mei-41 mutants caused by transposase-induced DSBs suggests that this defect is primarily or entirely due to loss of cell-cycle regulation. As a second test of this hypothesis, we examined repair and survival in grp and lok mutants. The grp and lok genes encode orthologs of Chk1 and Chk2, respectively. In other organisms, Chk1 and Chk2 have partially redundant roles in transducing cell-cycle checkpoints (Boddy et al. 1998; Chen and Sanchez 2004; Helt et al. 2005; reviewed in Sanchez et al. 1996; Sancar et al. 2004). Drosophila grp and lok mutants are defective in replication and damage checkpoints (Su et al. 1999; Yu et al. 2000; Masrouha et al. 2003; Brodsky et al. 2004; Jaklevic and Su 2004; de Vries et al. 2005; Royou et al. 2005). In some studies, grp mutants were found to completely lack the G2-M DNA damage checkpoint (Jaklevic and Su 2004; de Vries et al. 2005), but others have suggested that GRP and LOK have partially redundant functions in this response (Xu et al. 2001; Brodsky et al. 2004).
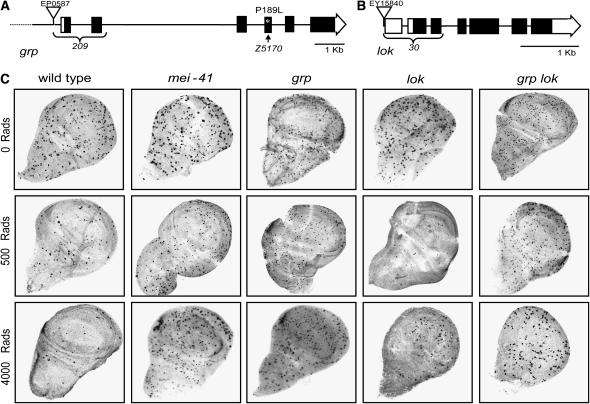
We could not use existing alleles of grp and lok because they have P-element insertions, so we generated and characterized new mutations in grp (grp209 and grpZ5170) and lok (lok30; see Figure 5 and materials and methods for details). The grp209 and grpZ5170 alleles were screened for failure to complement the maternal-effect embryonic lethal phenotype of grp1, which has been proposed to be null (Bier et al. 1989; Fogarty et al. 1994). Consistent with previous results with null alleles, the G2-M checkpoint induced by 4000 rad of IR was decreased in lok30 mutants (Xu et al. 2001) and was not detectable in grp209/grpZ5170 mutants (Jaklevic and Su 2004; Figure 5C). As with other alleles, double mutants of these alleles are indistinguishable from mei-41 mutants in that the G2-M checkpoint was undetectable after 4000 rad of IR (Brodsky et al. 2004). At lower doses (500 rad), a checkpoint response was undetectable in both grp single and grp lok double mutants, but was not affected in lok single mutants (Figure 5C). On the basis of molecular data and similarity to previously characterized null alleles of these genes, we conclude that these new alleles are genetically null.
Figure 5.—
Checkpoint defects in grp and lok mutants. (A) Schematic of the genomic architecture of grp is shown. Each box represents an exon; solid regions designate protein-coding regions (the four alternative first exons, which are noncoding, are not shown). grp209 was generated by excision of EP587 (triangle) to generate a deletion that removes the first two coding exons (brace). grpZ5170 has a C-to-T transition that changes a conserved proline at residue 189 in the kinase domain to leucine (asterisk; see materials and methods for details). (B) The lok30 allele was generated by excision of EY15840 (triangle), generating a deletion of the 5′-UTR and the first two coding exons (brace). (C) DNA damage checkpoint defects in mutants. Third instar larvae of the indicated genotype were unirradiated (top) or irradiated with either 500 rad (middle) or 4000 rad (bottom) of γ-rays. Imaginal discs were dissected and fixed 1 hr after irradiation. Mitotic cells are revealed by staining with an antibody to phosphorylated histone H3.
We used these mutations to determine whether the SDSA defects observed in mei-41 mutants are due to a loss of the checkpoint response. We first investigated lethality associated with P-element excision. Because grp and lok are on chromosome 2, we used a chromosome 3 transposase source. This source is less active, resulting in fewer excisions, but this does not affect repair outcome (McVey et al. 2004a; this study). To offset the decreased transposase activity, we measured viability in the presence of an increased number of P elements: a combination of snw, which is an insertion of two small P elements, and the original P{wa} element. For comparison, we also tested genotypes assayed in Figure 1A with this combination of P elements and transposase. As in the previous assay, viability of mei-41 mutants is strongly decreased by the presence of transposase (Figure 1B). In contrast, grp and lok single mutants were fully viable. In grp lok double mutants, viability was decreased relative to wild type or either single mutant, but the decrease was not as severe as in mei-41 mutants (P < 0.05). Thus, while reducing cyclins can completely rescue lethality in mei-41 mutants, loss of the GRP/LOK-dependent checkpoint can account for only part of the viability defect of mei-41 mutants.
We also assayed grp and lok mutants for specific repair defects in the germline. Although neither single mutant had a detectable defect in completing SDSA, the double mutant exhibited a significant (P < 0.05) decrease in the ability to complete SDSA (Figure 3); however, the decrease was not as severe as that of mei-41 mutants (P < 0.05). Similarly, grp and lok single mutants did not show a decreased ability to repair through SSA, but the double mutant had a phenotype intermediate between that of wild type and mei-41 mutants (Table 1; P < 0.001). Among aborted SDSA repair events, synthesis tract lengths were similar in grp and lok single mutants, as well as in grp lok double mutants, to those of wild type and mei-41 mutants (data not shown). Thus, loss of both GRP and LOK generates repair defects that are qualitatively identical to, but quantitatively less severe than, those resulting from loss of MEI-41. These results support the conclusion that loss of the DNA damage-dependent checkpoint mediated through GRP and LOK is only partially responsible for the SDSA repair in mei-41 mutants.
DISCUSSION
In this study, we addressed two aspects of the role of MEI-41 in DSB repair: (1) which specific DSB repair processes are influenced by loss of MEI-41 and (2) whether the checkpoint function of MEI-41 accounts for defects in repair observed in mei-41 mutants. Our results indicate that loss of MEI-41 affects repair by HR, but not by NHEJ, and that the disruption of HR cannot be explained entirely by loss of the GRP/LOK-mediated G2-M DNA damage checkpoint. This suggests that MEI-41 regulates repair through a mechanism independent of the GRP/LOK-mediated checkpoint response.
Evidence that the effect of loss of MEI-41 is specific to HR comes from our finding that lethality of mei-41 mutants undergoing P-element excision is eliminated through use of spn-A mutations. A previous study suggested that repair of DSBs generated by P-element excision in somatic tissues occurs primarily through NHEJ (Gloor et al. 2000), but our results indicate that repair by HR is also important, at least in some essential tissue or tissues. This conclusion was also reached by Romeijn et al. (2005), who demonstrated that mutations in okra, which encodes the ortholog of Rad54, also impart sensitivity to DSBs generated through P-element excision. In our assay, however, spn-A mutants, which are not capable of repairing through HR, are fully viable. The difference between this result and that of Romeijn et al. is probably due to different rates of excision of the different P-element insertions used. Consistent with this interpretation, we have observed that differences in the rate of excision of the same P{wa} insertion, resulting from the use of different sources of transposase, can affect viability of repair-defective mutants (McVey et al. 2004a; this study).
Defects in HR, but not in NHEJ, were also revealed through analysis of germline repair events: Recovery of products arising from completed SDSA was reduced in mei-41 mutants, but there was no effect on recovery of products in which repair was completed by end joining. The latter type of product appears to arise from initiation of SDSA with multiple rounds of strand invasion, repair synthesis, and dissociation of the nascent strand. In these products, however, repair is completed through a DNA ligase IV-independent end-joining pathway (Figure 2i) (McVey et al. 2004a,b), rather than by annealing of complementary strands, as in SDSA. Because products in this class are indistinguishable between mei-41 mutants and wild-type males, we conclude that loss of MEI-41 does not affect the ability to repair through end joining, but rather the later steps in SDSA (Figure 2h).
Much of the effect of loss of MEI-41 on repair can be attributed to the cell-cycle checkpoint function. Reduced viability of mei-41 mutants undergoing P-element excision can be completely suppressed by reducing levels of mitotic cyclins. One interpretation of this result is that reducing cyclins delays entry into or progression through mitosis, thereby alleviating the need for cell-cycle regulation by MEI-41. Another possibility is that cyclin reduction affects viability in some way other than through cell-cycle regulation. Ira et al. (2004) found that inhibiting Cdk1 (required for cell-cycle progression into mitosis) in budding yeast resulted in increased NHEJ repair of an HO endonuclease-induced DSB. It is possible that the cyclin reduction in our experiments increases repair through NHEJ, resulting in an effect similar to that of eliminating SPN-A. This was not true in the male germline, where the fractions of different repair products in CycB/+ or CycA/+ mutants were not different from those in wild-type flies (data not shown). In the male germline, however, reducing the zygotic dose of these cyclins did not affect the reduced ability of mei-41 mutants to complete repair by SDSA (data not shown). This result may reflect different effects of cyclin reductions on cell-cycle progression into mitosis in different tissues and therefore does not provide insights into the mechanism by which cyclin reductions rescue the lethality of mei-41 mutants experiencing excision of P elements during development.
Additional support for an important role for MEI-41-dependent cell-cycle regulation in the ability to repair DSBs through SDSA comes from analysis of mutants lacking GRP and LOK, which are required for the replication and DNA damage checkpoint response in Drosophila (Su et al. 1999; Yu et al. 2000; Masrouha et al. 2003; Brodsky et al. 2004; Jaklevic and Su 2004; de Vries et al. 2005; Royou et al. 2005). We found that grp lok double mutants had the same phenotypes as mei-41 mutants. However, the effects were less severe in each of three different assays (viability, SDSA repair, and SSA repair).
The IR-induced G2-M checkpoint is undetectable in grp single mutants (as in mei-41 mutants) and is partially defective in lok single mutants at high doses (Liu et al. 2000; Xu et al. 2001; Brodsky et al. 2004; this study); however, we did not detect repair defects in grp or lok single mutants. We attribute this apparent paradox to differences in the assays used. In Drosophila studies, the G2-M checkpoint is observed after exposure to IR, by staining proliferating imaginal disc tissues with a marker for mitosis (an antibody to phosphorylated histone H3). In our assays, we looked for defects in HR repair of DSBs generated by P-element excision in different tissues, throughout development. P-element excision leaves a 17-nt 3′-ended single-strand overhang (Beall and Rio 1997), whereas IR causes multiple types of damage, including single-strand nicks and DSBs (Hutchinson 1985; Frankenberg-Schwager 1990; Ward 1990; Price 1993). There may also be tissue-specific differences in the requirements for GRP and LOK for checkpoint signaling. For example, GRP may be essential for this process in imaginal disc cells, but redundant with LOK in the male germline. It is also interesting that no requirement for LOK is observed at low doses of IR. This suggests that LOK is activated only at high doses to facilitate the G2-M checkpoint. Finally, we cannot eliminate the possibility that GRP and LOK may be redundant in a checkpoint-independent pathway that contributes to SDSA repair, but there is at present no evidence that these proteins act redundantly in DNA damage repair pathways in Drosophila or other organisms.
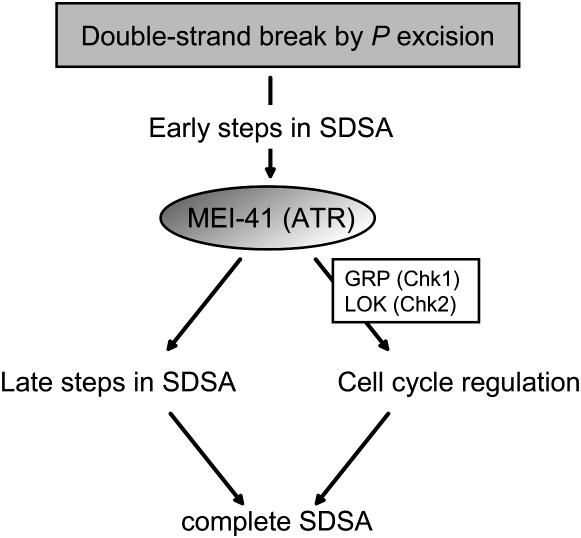
All of the effects of loss of MEI-41 on ability to complete SDSA may be due to loss of the DNA damage-dependent checkpoint, if this checkpoint is not completely eliminated in grp lok double mutants. In the assay we and others use to assess the G2-M checkpoint, the phenotype of grp lok double mutants is indistinguishable from that of mei-41 mutants (Liu et al. 2000; Xu et al. 2001; Brodsky et al. 2004; this study), but it is possible that some effects of the checkpoint are not detectable with this assay. If grp lok double mutants retain some aspect of the MEI-41-dependent DNA damage checkpoint, there must be some transducer that remains to be identified. Studies in other model organisms suggest that ATR/ATM-dependent DNA damage checkpoints are transduced entirely through Chk1 and Chk2 (Boddy et al. 1998; Chen and Sanchez 2004; reviewed in Sanchez et al. 1996; Sancar et al. 2004). In Xenopus, Mcm2 is a direct substrate of ATM and ATR in response to DNA damage in the DNA replication checkpoint (Yoo et al. 2004). However, repair of the P-excision-induced DSB analyzed in this study requires a sister chromatid for HR and therefore must occur in G2 (see results). We suggest that the G2-M DNA damage checkpoint function of MEI-41 accounts for only a portion of the reduced ability to complete SDSA when MEI-41 is absent and that MEI-41 has a role in response to DNA damage independent of the GRP/LOK-mediated checkpoint (Figure 6).
Figure 6.—
Model for MEI-41 in repair of DSBs. We propose that MEI-41 is involved in the later steps of SDSA in repairing a single DSB (after strand invasion and repair synthesis). Our genetic experiments indicate that in addition to the GRP/LOK-dependent checkpoint response, MEI-41 is also involved in repairing DSBs independent of this checkpoint. The presence of MEI-41 in both of these pathways ensures complete repair through SDSA.
In conclusion, our results indicate that MEI-41 has multiple roles in regulating responses to DNA damage. We found that mei-41 mutants are compromised in their ability to complete the later steps of SDSA, but not in NHEJ. The defects seem to be due in part to loss of the GRP/LOK-mediated G2-M DNA damage checkpoint, resulting in insufficient time for repair in some cells. This suggests that the later steps in SDSA—annealing of complementary sequences, trimming overhangs and/or filling gaps, and ligation—are more time-consuming than joining the ends generated by multiple cycles of repair synthesis. Our finding that the GRP/LOK-mediated checkpoint function of MEI-41 does not entirely account for the defects of mei-41 mutants in HR repair implies that MEI-41 has an additional function in facilitating the later stages of HR repair that is independent of this checkpoint, as suggested in studies examining other functions of MEI-41 (Jaklevic and Su 2004; Oikemus et al. 2006). It has been suggested that mammalian ATM kinases have a role in DSB repair independent of their checkpoint functions (reviewed in Jeggo et al. 1998; Lobrich and Jeggo 2005; Jeggo and Lobrich 2006; O'Driscoll and Jeggo 2006). Given the conservation of checkpoint functions between these kinases and MEI-41, it may be that mammalian ATM kinases facilitate HR in a manner similar to the function of MEI-41 described here.
Acknowledgments
We thank Alysia Kern for collection of preliminary data, Mitch McVey and Melissa D. Adams for development of and expertise regarding the P{wa} assay, and members of the Sekelsky lab for helpful discussions. We are grateful to Li Lin of the Lineberger Comprehensive Cancer Center's Biostatistics and Data Management Facility for statistical analyses. J.R.L. was supported by a Graduate Research Fellowship from the National Science Foundation. This research was supported by grants from the National Institutes of Health (GM66441) to T.T.S. and from the American Cancer Society (RSG-05-138-01-GMC) to J.J.S.
References
- Abraham, R. T., 2001. Cell cycle checkpoint signaling through the ATM and ATR kinases. Genes Dev. 15: 2177–2196. [DOI] [PubMed] [Google Scholar]
- Adams, M. D., M. McVey and J. Sekelsky, 2003. Drosophila BLM in double-strand break repair by synthesis-dependent strand annealing. Science 299: 265–267. [DOI] [PubMed] [Google Scholar]
- Banga, S. S., A. Velazquez and J. B. Boyd, 1991. P transposition in Drosophila provides a new tool for analyzing postreplication repair and double-strand break repair. Mutat. Res. 255: 79–88. [DOI] [PubMed] [Google Scholar]
- Bassing, C. H., K. F. Chua, J. Sekiguchi, H. Suh, S. R. Whitlow et al., 2002. Increased ionizing radiation sensitivity and genomic instability in the absence of histone H2AX. Proc. Natl. Acad. Sci. USA 99: 8173–8178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beall, E. L., and D. C. Rio, 1997. Drosophila P-element transposase is a novel site-specific endonuclease. Genes Dev. 11: 2137–2151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bi, X., S. C. Wei and Y. S. Rong, 2004. Telomere protection without a telomerase; the role of ATM and Mre11 in Drosophila telomere maintenance. Curr. Biol. 14: 1348–1353. [DOI] [PubMed] [Google Scholar]
- Bi, X., M. Gong, D. Srikanta and Y. S. Rong, 2005. a Drosophila ATM and Mre11 are essential for the G2/M checkpoint induced by low-dose irradiation. Genetics 171: 845–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bi, X., D. Srikanta, L. Fanti, S. Pimpinelli, R. Badugu et al., 2005. b Drosophila ATM and ATR checkpoint kinases control partially redundant pathways for telomere maintenance. Proc. Natl. Acad. Sci. USA 102: 15167–15172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bier, E., H. Vaessin, S. Shepherd, K. Lee, K. McCall et al., 1989. Searching for pattern and mutation in the Drosophila genome with P-lacZ vector. Genes Dev. 3: 1273–1287. [DOI] [PubMed] [Google Scholar]
- Boddy, M. N., B. Furnari, O. Mondesert and P. Russell, 1998. Replication checkpoint enforced by kinases Cds1 and Chk1. Science 280: 909–912. [DOI] [PubMed] [Google Scholar]
- Boyd, J. B., M. D. Golino, T. D. Nguyen and M. M. Green, 1976. Isolation and characterization of X-linked mutants of Drosophila melanogaster which are sensitive to mutagens. Genetics 84: 485–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodsky, M. H., J. Sekelsky, G. Tsang, R. S. Hawley and G. M. Rubin, 2000. mus304 encodes a novel DNA damage checkpoint protein required during Drosophila development. Genes Dev. 14: 666–678. [PMC free article] [PubMed] [Google Scholar]
- Brodsky, M. H., B. T. Weinert, G. Tsang, Y. S. Rong, N. M. McGinnis et al., 2004. Drosophila melanogaster MNK/Chk2 and p53 regulate multiple DNA repair and apoptotic pathways following DNA damage. Mol. Cell. Biol. 24: 1219–1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown, E. J., and D. Baltimore, 2003. Essential and dispensable roles of ATR in cell cycle arrest and genome maintenance. Genes Dev. 17: 615–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canman, C. E., D.-S. Lin, K. A. Cimprich, Y. Taya, K. Tamai et al., 1998. Activation of the ATM kinase by ionizing radiation and phosphorylation of p53. Science 281: 1677–1679. [DOI] [PubMed] [Google Scholar]
- Celeste, A., S. Petersen, P. J. Romanienko, O. Fernandez-Capetillo, H. T. Chen et al., 2002. Genomic instability in mice lacking histone H2AX. Science 296: 922–927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, Y., and Y. Sanchez, 2004. Chk1 in the DNA damage response: conserved roles from yeasts to mammals. DNA Repair 3: 1025–1032. [DOI] [PubMed] [Google Scholar]
- Cliby, W. A., C. J. Roberts, K. A. Cimprich, C. M. Stringer, J. R. Lamb et al., 1998. Overexpression of a kinase-inactive ATR protein causes sensitivity to DNA-damaging agents and defects in cell cycle checkpoints. EMBO J. 17: 159–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das, K. C., and R. Dashnamoorthy, 2004. Hyperoxia activates the ATR-Chk1 pathway and phosphorylates p53 at multiple sites. Am. J. Physiol. Lung Cell Mol. Physiol. 286: L87–L97. [DOI] [PubMed] [Google Scholar]
- de Vries, H. I., L. Uyetake, W. Lemstra, J. F. Brunsting, T. T. Su et al., 2005. Grp/DChk1 is required for G2-M checkpoint activation in Drosophila S2 cells, whereas Dmnk/DChk2 is dispensable. J. Cell Sci. 118: 1833–1842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgar, B. A., F. Sprenger, R. J. Duronio, P. Leopold and P. H. O′Farrell, 1994. Distinct molecular mechanisms regulate cell cycle timing at successive stages of Drosophila embryogenesis. Genes Dev. 8: 440–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engels, W. R., W. K. Benz, C. R. Preston, P. L. Graham, R. W. Phillis et al., 1987. Somatic effects of P element activity in Drosophila melanogaster: pupal lethality. Genetics 117: 745–757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fogarty, P., R. F. Kalpin and W. Sullivan, 1994. The Drosophila maternal-effect mutation grapes causes a metaphase arrest at nuclear cycle 13. Development 120: 2131–2142. [DOI] [PubMed] [Google Scholar]
- Fogarty, P., S. D. Campbell, R. Abu-Shumays, B. de Saint Phalle, K. R. Yu et al., 1997. The Drosophila grapes gene is related to checkpoint gene chk1/rad27 and is required for late syncytial division fidelity. Curr. Biol. 7: 418–426. [DOI] [PubMed] [Google Scholar]
- Frankenberg-Schwager, M., 1990. Induction, repair and biological relevance of radiation-induced DNA lesions in eukaryotic cells. Radiat. Environ. Biophys. 29: 273–292. [DOI] [PubMed] [Google Scholar]
- Garner, M., S. van Kreeveld and T. T. Su, 2001. mei-41 and bub1 block mitosis at two distinct steps in response to incomplete DNA replication in Drosophila embryos. Curr. Biol. 11: 1595–1599. [DOI] [PubMed] [Google Scholar]
- Gloor, G. B., J. Moretti, J. Mouyal and K. J. Keeler, 2000. Distinct P-element excision products in somatic and germline cells of Drosophila melanogaster. Genetics 155: 1821–1830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hari, K. L., A. Santerre, J. Sekelsky, K. S. McKim, J. B. Boyd et al., 1995. The mei-41 gene of D. melanogaster is a structural and functional homolog of the human ataxia telangiectasia gene. Cell 82: 815–821. [DOI] [PubMed] [Google Scholar]
- Helt, C. E., W. A. Cliby, P. C. Keng, R. A. Bambara and M. A. O′Reilly, 2005. Ataxia telangiectasia mutated (ATM) and ATM and Rad3-related protein exhibit selective target specificities in response to different forms of DNA damage. J. Biol. Chem. 280: 1186–1192. [DOI] [PubMed] [Google Scholar]
- Hutchinson, F., 1985. Chemical changes induced in DNA by ionizing radiation. Prog. Nucleic Acid Res. Mol. Biol. 32: 115–154. [DOI] [PubMed] [Google Scholar]
- Ira, G., A. Pellicioli, A. Balijja, X. Wang, S. Fiorani et al., 2004. DNA end resection, homologous recombination and DNA damage checkpoint activation require CDK1. Nature 431: 1011–1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanov, E. L., N. Sugawara, J. Fishman-Lobell and J. E. Haber, 1996. Genetic requirements for the single-strand annealing pathway for double-strand break repair in Saccharomyces cerevisiae. Genetics 142: 693–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs, H. W., J. A. Knoblich and C. F. Lehner, 1998. Drosophila Cyclin B3 is required for female fertility and is dispensable for mitosis like Cyclin B. Genes Dev. 12: 3741–3751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaklevic, B. R., and T. T. Su, 2004. Relative contribution of DNA repair, cell cycle checkpoints, and cell death to survival after DNA damage in Drosophila larvae. Curr. Biol. 14: 23–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeggo, P. A., and M. Lobrich, 2005. Artemis links ATM to double strand break rejoining. Cell Cycle 4: 359–362. [DOI] [PubMed] [Google Scholar]
- Jeggo, P. A., and M. Lobrich, 2006. Contribution of DNA repair and cell cycle checkpoint arrest to the maintenance of genomic stability. DNA Repair 5: 1192–1198. [DOI] [PubMed] [Google Scholar]
- Jeggo, P. A., A. M. Carr and A. R. Lehmann, 1998. Splitting the ATM: distinct repair and checkpoint defects in ataxia-telangiectasia. Trends Genet. 14: 312–316. [DOI] [PubMed] [Google Scholar]
- Koundakjian, E. J., D. M. Cowan, R. W. Hardy and A. H. Becker, 2004. The Zuker collection: a resource for the analysis of autosomal gene function in Drosophila melanogaster. Genetics 167: 203–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhne, M., E. Riballo, N. Rief, K. Rothkamm, P. A. Jeggo et al., 2004. A double-strand break repair defect in ATM-deficient cells contributes to radiosensitivity. Cancer Res. 64: 500–508. [DOI] [PubMed] [Google Scholar]
- Kurkulos, M., J. M. Weinberg, D. Roy and S. M. Mount, 1994. P element-mediated in vivo deletion analysis of white-apricot: deletions between direct repeats are strongly favored. Genetics 136: 1001–1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurencon, A., A. Purdy, J. Sekelsky, R. S. Hawley and T. T. Su, 2003. Phenotypic analysis of separation-of-function alleles of MEI-41, Drosophila ATM/ATR. Genetics 164: 589–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, Q., S. Guntuku, X. S. Cui, S. Matsuoka, D. Cortez et al., 2000. Chk1 is an essential kinase that is regulated by Atr and required for the G(2)/M DNA damage checkpoint. Genes Dev. 14: 1448–1459. [PMC free article] [PubMed] [Google Scholar]
- Lobrich, M., and P. A. Jeggo, 2005. Harmonising the response to DSBs: a new string in the ATM bow. DNA Repair 4: 749–759. [DOI] [PubMed] [Google Scholar]
- Masrouha, N., L. Yang, S. Hijal, S. Larochelle and B. Suter, 2003. The Drosophila chk2 gene loki is essential for embryonic DNA double-strand-break checkpoints induced in S phase or G2. Genetics 163: 973–982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McVey, M., M. D. Adams, E. Staeva-Vieira and J. Sekelsky, 2004. a Evidence for multiple cycles of strand invasion during repair of double-strand gaps in Drosophila. Genetics 167: 699–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McVey, M., D. Radut and J. Sekelsky, 2004. b End-joining repair of double-strand breaks in Drosophila is largely DNA ligase IV-independent. Genetics 168: 2067–2076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills, K. D., D. A. Sinclair and L. Guarente, 1999. MEC1-dependent redistribution of the Sir3 silencing protein from telomeres to DNA double-strand breaks. Cell 97: 609–620. [DOI] [PubMed] [Google Scholar]
- Morrison, C., E. Sonoda, N. Takao, A. Shinohara, K. Yamamoto et al., 2000. The controlling role of ATM in homologous recombinational repair of DNA damage. EMBO J. 19: 463–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nassif, N., and W. R. Engels, 1993. DNA homology requirements for mitotic gap repair in Drosophila. Proc. Natl. Acad. Sci. USA 90: 1262–1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Driscoll, M., and P. A. Jeggo, 2006. The role of double-strand break repair—insights from human genetics. Nat. Rev. Genet. 7: 45–54. [DOI] [PubMed] [Google Scholar]
- Oikemus, S. R., J. Queiroz-Machado, K. Lai, N. McGinnis, C. Sunkel et al., 2006. Epigenetic telomere protection by Drosophila DNA damage response pathways. PLoS Genet. 2: e71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paull, T. T., E. P. Rogakou, V. Yamazaki, C. U. Kirchgessner, M. Gellert et al., 2000. A critical role for histone H2AX in recruitment of repair factors to nuclear foci after DNA damage. Curr. Biol. 10: 886–895. [DOI] [PubMed] [Google Scholar]
- Price, A., 1993. The repair of ionising radiation-induced damage to DNA. Semin. Cancer Biol. 4: 61–71. [PubMed] [Google Scholar]
- Riballo, E., M. Kuhne, N. Rief, A. Doherty, G. C. Smith et al., 2004. A pathway of double-strand break rejoining dependent upon ATM, Artemis, and proteins locating to gamma-H2AX foci. Mol. Cell 16: 715–724. [DOI] [PubMed] [Google Scholar]
- Romeijn, R. J., M. M. Gorski, M. A. van Schie, J. N. Noordermeer, L. H. Mullenders et al., 2005. Lig4 and rad54 are required for repair of DNA double-strand breaks induced by P-element excision in Drosophila. Genetics 169: 795–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rong, Y. S., and K. G. Golic, 2000. Gene targeting by homologous recombination in Drosophila. Science 288: 2013–2018. [DOI] [PubMed] [Google Scholar]
- Royou, A., H. Macias and W. Sullivan, 2005. The Drosophila Grp/Chk1 DNA damage checkpoint controls entry into anaphase. Curr. Biol. 15: 334–339. [DOI] [PubMed] [Google Scholar]
- Sancar, A., L. A. Lindsey-Boltz, K. Unsal-Kacmaz and S. Linn, 2004. Molecular mechanisms of mammalian DNA repair and the DNA damage checkpoints. Annu. Rev. Biochem. 73: 39–85. [DOI] [PubMed] [Google Scholar]
- Sanchez, Y., B. A. Desany, W. J. Jones, Q. Liu, B. Wang et al., 1996. Regulation of RAD53 by the ATM-like kinases MEC1 and TEL1 in yeast cell cycle checkpoint pathways. Science 271: 357–360. [DOI] [PubMed] [Google Scholar]
- Shiloh, Y., 2003. ATM: ready, set, go. Cell Cycle 2: 116–117. [DOI] [PubMed] [Google Scholar]
- Sibon, O. C., A. Laurencon, R. Hawley and W. E. Theurkauf, 1999. The Drosophila ATM homologue Mei-41 has an essential checkpoint function at the midblastula transition. Curr. Biol. 9: 302–312. [DOI] [PubMed] [Google Scholar]
- Sigrist, S. J., and C. F. Lehner, 1997. Drosophila fizzy-related down-regulates mitotic cyclins and is required for cell proliferation arrest and entry into endocycles. Cell 90: 671–681. [DOI] [PubMed] [Google Scholar]
- Silva, E., S. Tiong, M. Pedersen, E. Homola, A. Royou et al., 2004. ATM is required for telomere maintenance and chromosome stability during Drosophila development. Curr. Biol. 14: 1341–1347. [DOI] [PubMed] [Google Scholar]
- Song, Y. H., G. Mirey, M. Betson, D. A. Haber and J. Settleman, 2004. The Drosophila ATM ortholog, dATM, mediates the response to ionizing radiation and to spontaneous DNA damage during development. Curr. Biol. 14: 1354–1359. [DOI] [PubMed] [Google Scholar]
- Staeva-Vieira, E., S. Yoo and R. Lehmann, 2003. An essential role of DmRad51/SpnA in DNA repair and meiotic checkpoint control. EMBO J. 22: 5863–5874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su, T. T., S. D. Campbell and P. H. O′Farrell, 1999. Drosophila grapes/CHK1 mutants are defective in cyclin proteolysis and coordination of mitotic events. Curr. Biol. 9: 919–922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor, A. M. R., D. G. Harnden, C. F. Arlett, C. F. Harcourt, A. R. Lehmann et al., 1975. Ataxia telangiectasia: a human mutation with abnormal radiation sensitivity. Nature 258: 427–429. [DOI] [PubMed] [Google Scholar]
- Unsal-Kacmaz, K., A. M. Makhov, J. D. Griffith and A. Sancar, 2002. Preferential binding of ATR protein to UV-damaged DNA. Proc. Natl. Acad. Sci. USA 99: 6673–6678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, X., J. Khadpe, B. Hu, G. Iliakis and Y. Wang, 2003. An overactivated ATR/CHK1 pathway is responsible for the prolonged G2 accumulation in irradiated AT cells. J. Biol. Chem. 278: 30869–30874. [DOI] [PubMed] [Google Scholar]
- Ward, I. M., and J. Chen, 2001. Histone H2AX is phosphorylated in an ATR-dependent manner in response to replicational stress. J. Biol. Chem. 276: 47759–47762. [DOI] [PubMed] [Google Scholar]
- Ward, J. F., 1990. The yield of DNA double-strand breaks produced intracellularly by ionizing radiation: a review. Int. J. Radiat. Biol. 57: 1141–1150. [DOI] [PubMed] [Google Scholar]
- Wright, J. A., K. S. Keegan, D. R. Herendeen, N. J. Bentley, A. M. Carr et al., 1998. Protein kinase mutants of human ATR increase sensitivity to UV and ionizing radiation and abrogate cell cycle checkpoint control. Proc. Natl. Acad. Sci. USA 95: 7445–7450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu, J., S. Xin and W. Du, 2001. Drosophila Chk2 is required for DNA damage-mediated cell cycle arrest and apoptosis. FEBS Lett. 508: 394–398. [DOI] [PubMed] [Google Scholar]
- Yoo, H. Y., A. Shevchenko, A. Shevchenko and W. G. Dunphy, 2004. Mcm2 is a direct substrate of ATM and ATR during DNA damage and DNA replication checkpoint responses. J. Biol. Chem. 279: 53353–53364. [DOI] [PubMed] [Google Scholar]
- Yu, K. R., R. B. Saint and W. Sullivan, 2000. The Grapes checkpoint coordinates nuclear envelope breakdown and chromosome condensation. Nat. Cell Biol. 2: 609–615. [DOI] [PubMed] [Google Scholar]