Abstract
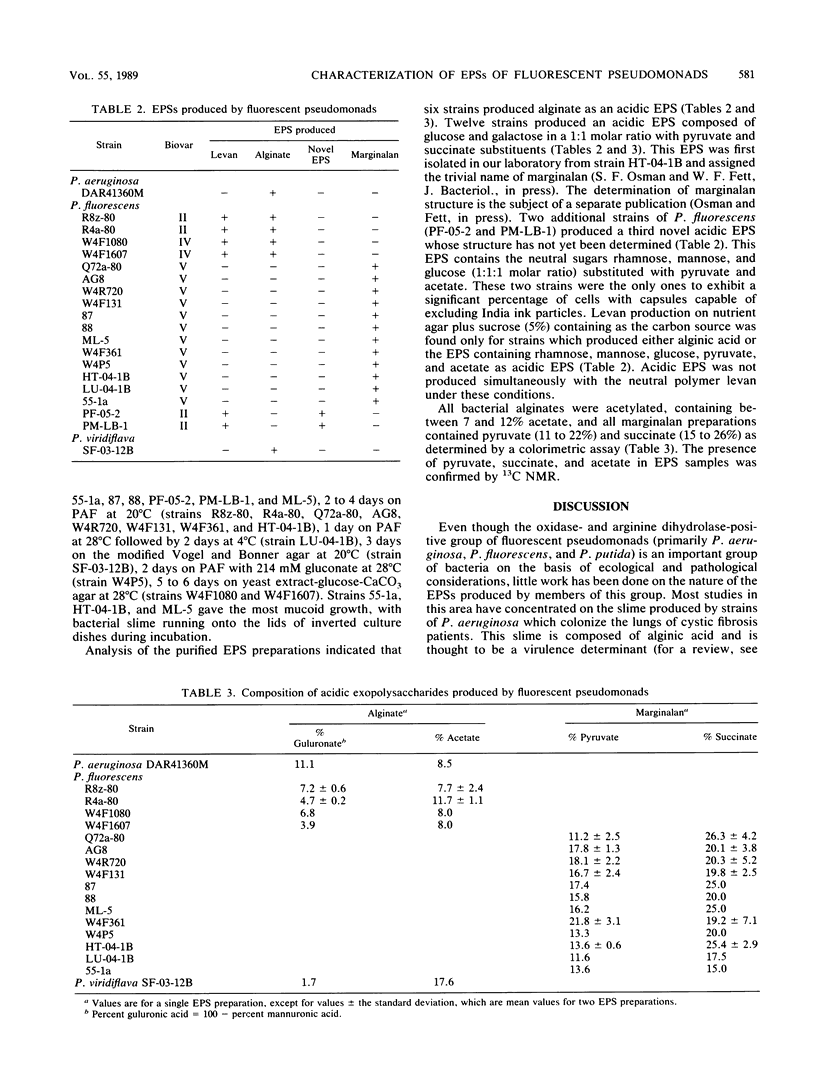
A total of 214 strains of plant-associated fluorescent pseudomonads were screened for the ability to produce the acidic exopolysaccharide (EPS) alginate on various solid media. The fluorescent pseudomonads studied were saprophytic, saprophytic with known biocontrol potential, or plant pathogenic. Approximately 10% of these strains exhibited mucoid growth under the conditions used. The EPSs produced by 20 strains were isolated, purified, and characterized. Of the 20 strains examined, 6 produced acetylated alginate as an acidic EPS. These strains included a Pseudomonas aeruginosa strain reported to cause a dry rot of onion, a strain of P. viridiflava with soft-rotting ability, and four strains of P. fluorescens. However, 12 strains of P. fluorescens produced a novel acidic EPS (marginalan) composed of glucose and galactose (1:1 molar ratio) substituted with pyruvate and succinate. Three of these strains were soft-rotting agents. Two additional soft-rotting strains of P. fluorescens produced a third acidic novel EPS composed of rhamnose, mannose, and glucose (1:1:1 molar ratio) substituted with pyruvate and acetate. When sucrose was present as the primary carbon source, certain strains produced the neutral polymer levan (a fructan) rather than an acidic EPS. Levan was produced by most strains capable of synthesizing alginate or the novel acidic EPS containing rhamnose, mannose, and glucose but not by strains capable of marginalan production. It is now evident that the group of bacteria belonging to the fluorescent pseudomonads is capable of elaborating a diverse array of acidic EPSs rather than solely alginate.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blumenkrantz N., Asboe-Hansen G. New method for quantitative determination of uronic acids. Anal Biochem. 1973 Aug;54(2):484–489. doi: 10.1016/0003-2697(73)90377-1. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Chan R., Lam J. S., Lam K., Costerton J. W. Influence of culture conditions on expression of the mucoid mode of growth of Pseudomonas aeruginosa. J Clin Microbiol. 1984 Jan;19(1):8–16. doi: 10.1128/jcm.19.1.8-16.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costerton J. W., Cheng K. J., Geesey G. G., Ladd T. I., Nickel J. C., Dasgupta M., Marrie T. J. Bacterial biofilms in nature and disease. Annu Rev Microbiol. 1987;41:435–464. doi: 10.1146/annurev.mi.41.100187.002251. [DOI] [PubMed] [Google Scholar]
- Duckworth M., Yaphe W. Definitive assay for pyruvic acid in agar and other algal polysaccharides. Chem Ind. 1970 Jun 6;23:747–748. [PubMed] [Google Scholar]
- Fett W. F., Osman S. F., Fishman M. L., Siebles T. S. Alginate production by plant-pathogenic pseudomonads. Appl Environ Microbiol. 1986 Sep;52(3):466–473. doi: 10.1128/aem.52.3.466-473.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Govan J. R., Fyfe J. A., Jarman T. R. Isolation of alginate-producing mutants of Pseudomonas fluorescens, Pseudomonas putida and Pseudomonas mendocina. J Gen Microbiol. 1981 Jul;125(1):217–220. doi: 10.1099/00221287-125-1-217. [DOI] [PubMed] [Google Scholar]
- Govan J. R., Harris G. S. Pseudomonas aeruginosa and cystic fibrosis: unusual bacterial adaptation and pathogenesis. Microbiol Sci. 1986 Oct;3(10):302–308. [PubMed] [Google Scholar]
- KING E. O., WARD M. K., RANEY D. E. Two simple media for the demonstration of pyocyanin and fluorescin. J Lab Clin Med. 1954 Aug;44(2):301–307. [PubMed] [Google Scholar]
- Osman S. F., Fett W. F., Fishman M. L. Exopolysaccharides of the phytopathogen Pseudomonas syringae pv. glycinea. J Bacteriol. 1986 Apr;166(1):66–71. doi: 10.1128/jb.166.1.66-71.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Read R. R., Costerton J. W. Purification and characterization of adhesive exopolysaccharides from Pseudomonas putida and Pseudomonas fluorescens. Can J Microbiol. 1987 Dec;33(12):1080–1090. doi: 10.1139/m87-189. [DOI] [PubMed] [Google Scholar]


