Abstract
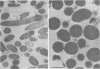
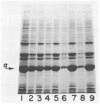
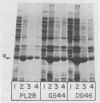
Certain Escherichia coli tryptophan synthase mutant alpha-subunits encoded from mutagenized trpA-containing plasmids were overexpressed as insoluble aggregates which were seen as large, intracellular inclusion bodies. The insoluble aggregates were solubilized to various degrees by several neutral, chaotropic salts. The order of effectiveness of these salts (KSCN, NaI greater than NaNO3, LiBr greater than CaCl2) followed that for the Hofmeister series. Optimum conditions for the use of KSCN resulted in a maximum 70 to 75% solubilization of the aggregate forms for all mutant alpha-subunits examined. Removal of KSCN by dialysis resulted in the recovery of biological activity and of certain characteristic structural properties. Such salts may be a useful alternative for other recombinant protein aggregates which resist complete renaturation by commonly used treatments with guanidine or urea.
Full text
PDF
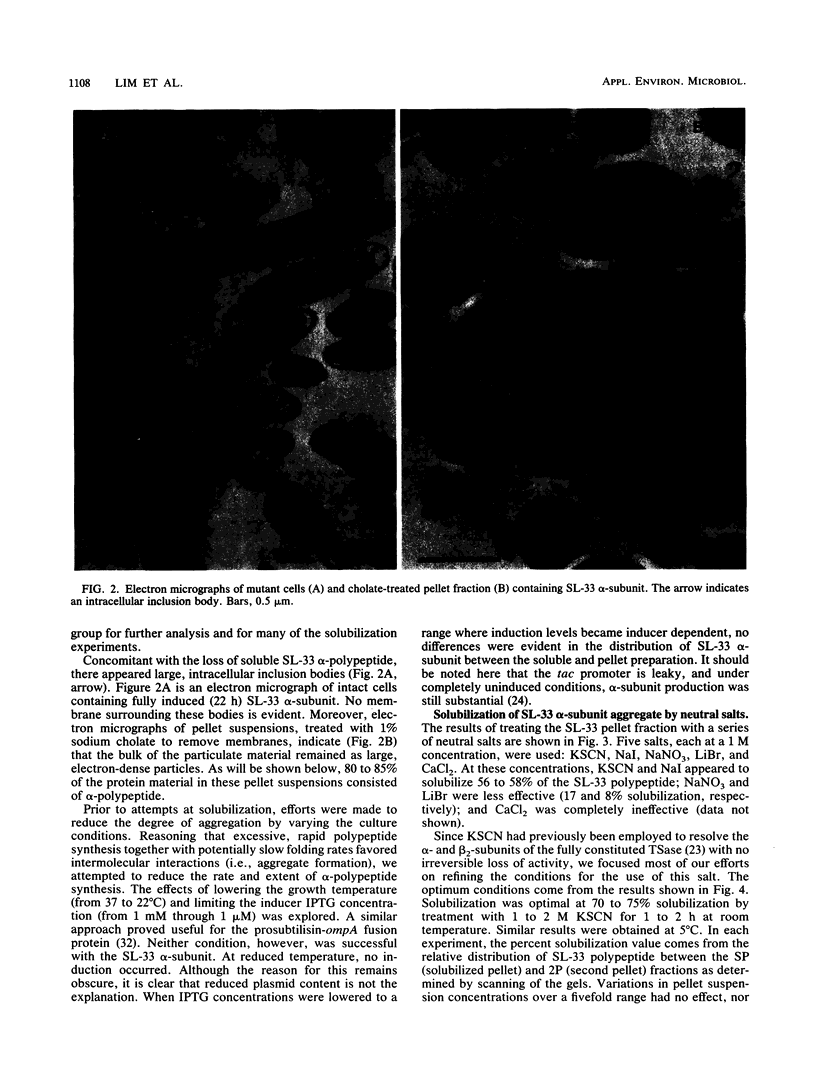
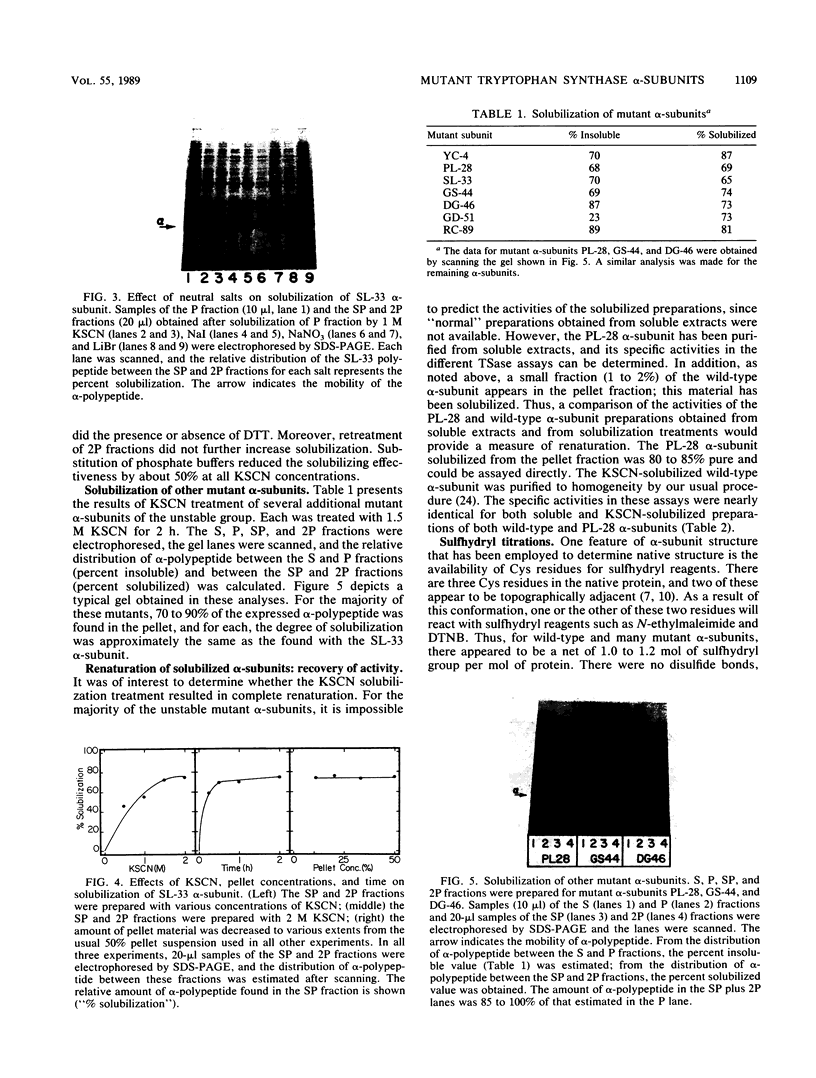
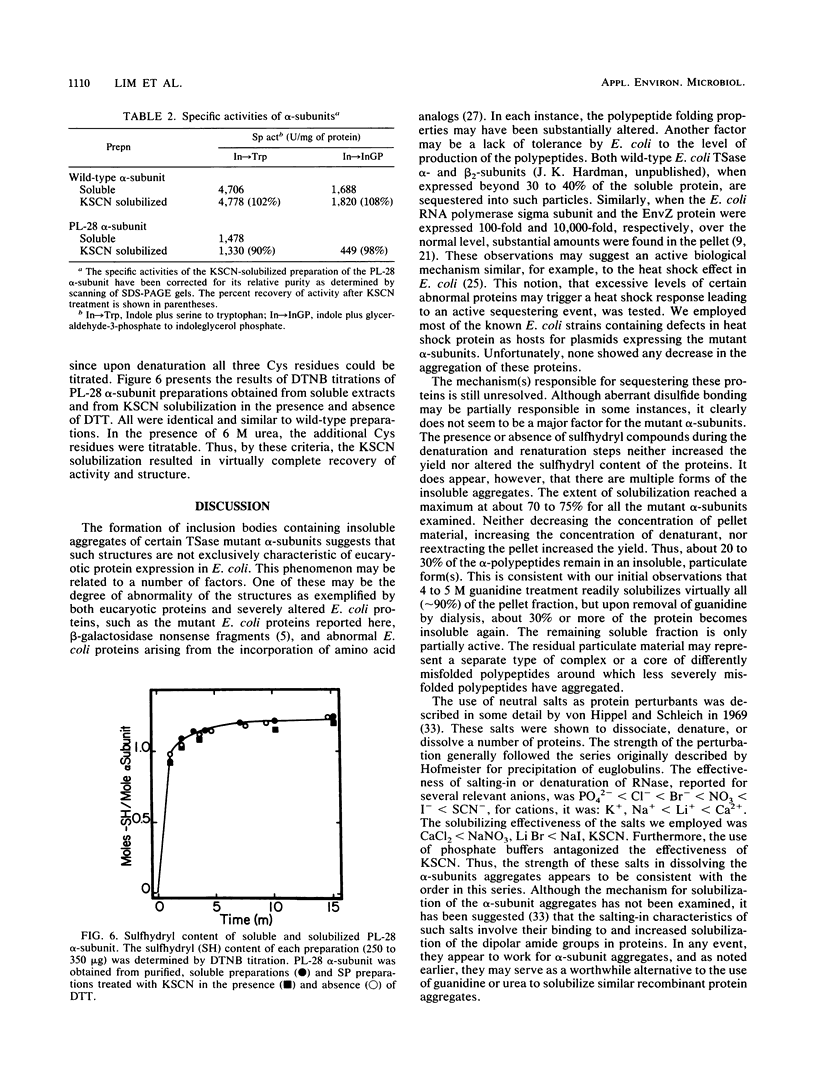

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adachi O., Kohn L. D., Miles E. W. Crystalline alpha2 beta2 complexes of tryptophan synthetase of Escherichia coli. A comparison between the native complex and the reconstituted complex. J Biol Chem. 1974 Dec 25;249(24):7756–7763. [PubMed] [Google Scholar]
- Amann E., Brosius J., Ptashne M. Vectors bearing a hybrid trp-lac promoter useful for regulated expression of cloned genes in Escherichia coli. Gene. 1983 Nov;25(2-3):167–178. doi: 10.1016/0378-1119(83)90222-6. [DOI] [PubMed] [Google Scholar]
- Bird R. E., Hardman K. D., Jacobson J. W., Johnson S., Kaufman B. M., Lee S. M., Lee T., Pope S. H., Riordan G. S., Whitlow M. Single-chain antigen-binding proteins. Science. 1988 Oct 21;242(4877):423–426. doi: 10.1126/science.3140379. [DOI] [PubMed] [Google Scholar]
- Boss M. A., Kenten J. H., Wood C. R., Emtage J. S. Assembly of functional antibodies from immunoglobulin heavy and light chains synthesised in E. coli. Nucleic Acids Res. 1984 May 11;12(9):3791–3806. doi: 10.1093/nar/12.9.3791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Y. S., Kwoh D. Y., Kwoh T. J., Soltvedt B. C., Zipser D. Stabilization of a degradable protein by its overexpression in Escherichia coli. Gene. 1981 Jun-Jul;14(1-2):121–130. doi: 10.1016/0378-1119(81)90154-2. [DOI] [PubMed] [Google Scholar]
- Devos R., Plaetinck G., Cheroutre H., Simons G., Degrave W., Tavernier J., Remaut E., Fiers W. Molecular cloning of human interleukin 2 cDNA and its expression in E. coli. Nucleic Acids Res. 1983 Jul 11;11(13):4307–4323. doi: 10.1093/nar/11.13.4307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freedberg W. B., Hardman J. K. Structural and functional roles of the cysteine residues in the alpha subunit of the Escherichia coli tryptophan synthetase. 3. Studies with the bifunctional sulfhydryl reagent bismaleimidomethyl ether. J Biol Chem. 1971 Mar 10;246(5):1439–1448. [PubMed] [Google Scholar]
- Goeddel D. V., Kleid D. G., Bolivar F., Heyneker H. L., Yansura D. G., Crea R., Hirose T., Kraszewski A., Itakura K., Riggs A. D. Expression in Escherichia coli of chemically synthesized genes for human insulin. Proc Natl Acad Sci U S A. 1979 Jan;76(1):106–110. doi: 10.1073/pnas.76.1.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gribskov M., Burgess R. R. Overexpression and purification of the sigma subunit of Escherichia coli RNA polymerase. Gene. 1983 Dec;26(2-3):109–118. doi: 10.1016/0378-1119(83)90180-4. [DOI] [PubMed] [Google Scholar]
- HARDMAN J. K., YANOFSKY C. STUDIES ON THE ACTIVE SITE OF THE A PROTEIN SUBUNIT OF THE ESCHERICHIA COLI TRYPTOPHAN SYNTHETASE. J Biol Chem. 1965 Feb;240:725–732. [PubMed] [Google Scholar]
- Ho Y., Lewis M., Rosenberg M. Purification and properties of a transcriptional activator. The cII protein of phage lambda. J Biol Chem. 1982 Aug 10;257(15):9128–9134. [PubMed] [Google Scholar]
- Itakura K., Hirose T., Crea R., Riggs A. D., Heyneker H. L., Bolivar F., Boyer H. W. Expression in Escherichia coli of a chemically synthesized gene for the hormone somatostatin. Science. 1977 Dec 9;198(4321):1056–1063. doi: 10.1126/science.412251. [DOI] [PubMed] [Google Scholar]
- Kirschner K., Wiskocil R. L., Foehn M., Rezeau L. The tryptophan synthase from Escherichia coli. An improved purification procedure for the alpha-subunit and binding studies with substrate analogues. Eur J Biochem. 1975 Dec 15;60(2):513–523. doi: 10.1111/j.1432-1033.1975.tb21030.x. [DOI] [PubMed] [Google Scholar]
- Kleid D. G., Yansura D., Small B., Dowbenko D., Moore D. M., Grubman M. J., McKercher P. D., Morgan D. O., Robertson B. H., Bachrach H. L. Cloned viral protein vaccine for foot-and-mouth disease: responses in cattle and swine. Science. 1981 Dec 4;214(4525):1125–1129. doi: 10.1126/science.6272395. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Langley K. E., Berg T. F., Strickland T. W., Fenton D. M., Boone T. C., Wypych J. Recombinant-DNA-derived bovine growth hormone from Escherichia coli. 1. Demonstration that the hormone is expressed in reduced form, and isolation of the hormone in oxidized, native form. Eur J Biochem. 1987 Mar 2;163(2):313–321. doi: 10.1111/j.1432-1033.1987.tb10802.x. [DOI] [PubMed] [Google Scholar]
- Malkinson A. M., Hardman J. K. Structural and functional roles of the cysteine residues in the alpha subunit of the Escherichia coli tryptophan synthetase. I. Structural roles and reactivity of the cysteine residues. Biochemistry. 1969 Jul;8(7):2769–2776. doi: 10.1021/bi00835a012. [DOI] [PubMed] [Google Scholar]
- Miles E. W., Bauerle R., Ahmed S. A. Tryptophan synthase from Escherichia coli and Salmonella typhimurium. Methods Enzymol. 1987;142:398–414. doi: 10.1016/s0076-6879(87)42051-x. [DOI] [PubMed] [Google Scholar]
- Miles E. W., Moriguchi M. Tryptophan synthase of Escherichia coli. Removal of pyridoxal 5'-phosphate and separation of the alpha and beta2 subunits. J Biol Chem. 1977 Oct 10;252(19):6594–6599. [PubMed] [Google Scholar]
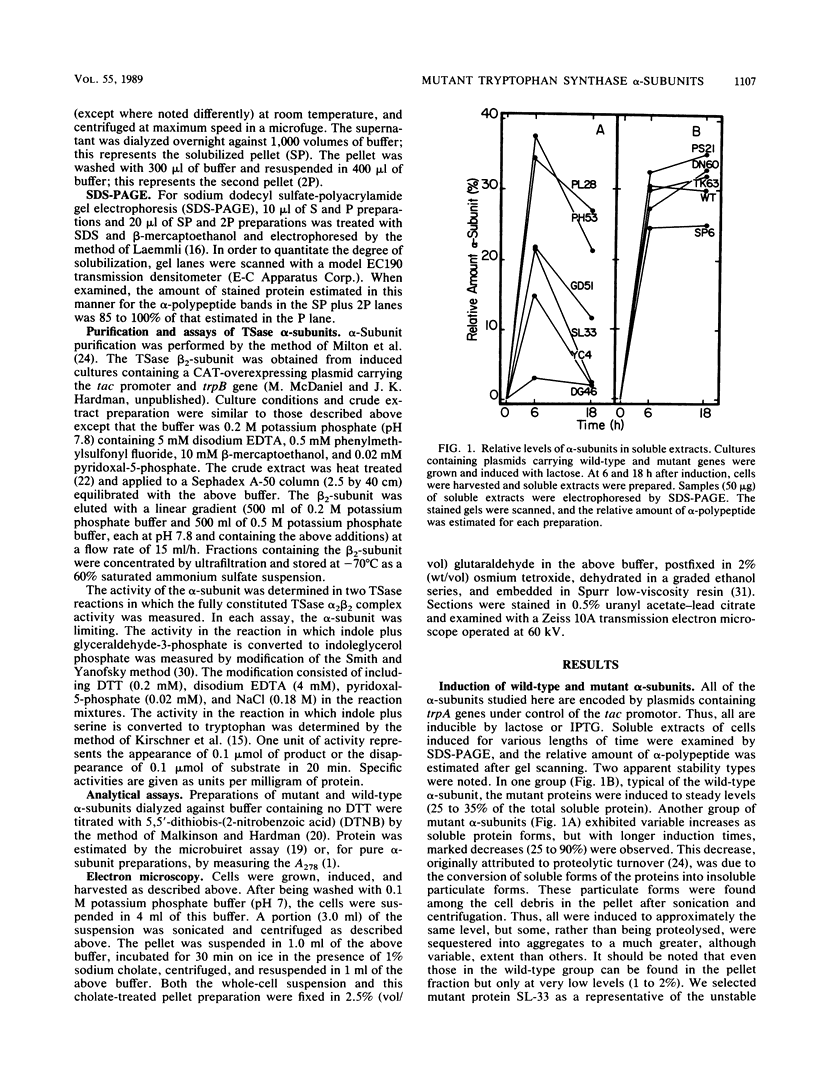
- Milton D. L., Napier M. L., Myers R. M., Hardman J. K. In vitro mutagenesis and overexpression of the Escherichia coli trpA gene and the partial characterization of the resultant tryptophan synthase mutant alpha-subunits. J Biol Chem. 1986 Dec 15;261(35):16604–16615. [PubMed] [Google Scholar]
- Neidhardt F. C., VanBogelen R. A., Vaughn V. The genetics and regulation of heat-shock proteins. Annu Rev Genet. 1984;18:295–329. doi: 10.1146/annurev.ge.18.120184.001455. [DOI] [PubMed] [Google Scholar]
- Paul D. C., Van Frank R. M., Muth W. L., Ross J. W., Williams D. C. Immunocytochemical demonstration of human proinsulin chimeric polypeptide within cytoplasmic inclusion bodies of Escherichia coli. Eur J Cell Biol. 1983 Sep;31(2):171–174. [PubMed] [Google Scholar]
- Prouty W. F., Karnovsky M. J., Goldberg A. L. Degradation of abnormal proteins in Escherichia coli. Formation of protein inclusions in cells exposed to amino acid analogs. J Biol Chem. 1975 Feb 10;250(3):1112–1122. [PubMed] [Google Scholar]
- Schoemaker J. M., Brasnett A. H., Marston F. A. Examination of calf prochymosin accumulation in Escherichia coli: disulphide linkages are a structural component of prochymosin-containing inclusion bodies. EMBO J. 1985 Mar;4(3):775–780. doi: 10.1002/j.1460-2075.1985.tb03696.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spurr A. R. A low-viscosity epoxy resin embedding medium for electron microscopy. J Ultrastruct Res. 1969 Jan;26(1):31–43. doi: 10.1016/s0022-5320(69)90033-1. [DOI] [PubMed] [Google Scholar]
- Williams D. C., Van Frank R. M., Muth W. L., Burnett J. P. Cytoplasmic inclusion bodies in Escherichia coli producing biosynthetic human insulin proteins. Science. 1982 Feb 5;215(4533):687–689. doi: 10.1126/science.7036343. [DOI] [PubMed] [Google Scholar]
- Winkler M. E., Bringman T., Marks B. J. The purification of fully active recombinant transforming growth factor alpha produced in Escherichia coli. J Biol Chem. 1986 Oct 15;261(29):13838–13843. [PubMed] [Google Scholar]
- van Kimmenade A., Bond M. W., Schumacher J. H., Laquoi C., Kastelein R. A. Expression, renaturation and purification of recombinant human interleukin 4 from Escherichia coli. Eur J Biochem. 1988 Apr 5;173(1):109–114. doi: 10.1111/j.1432-1033.1988.tb13973.x. [DOI] [PubMed] [Google Scholar]