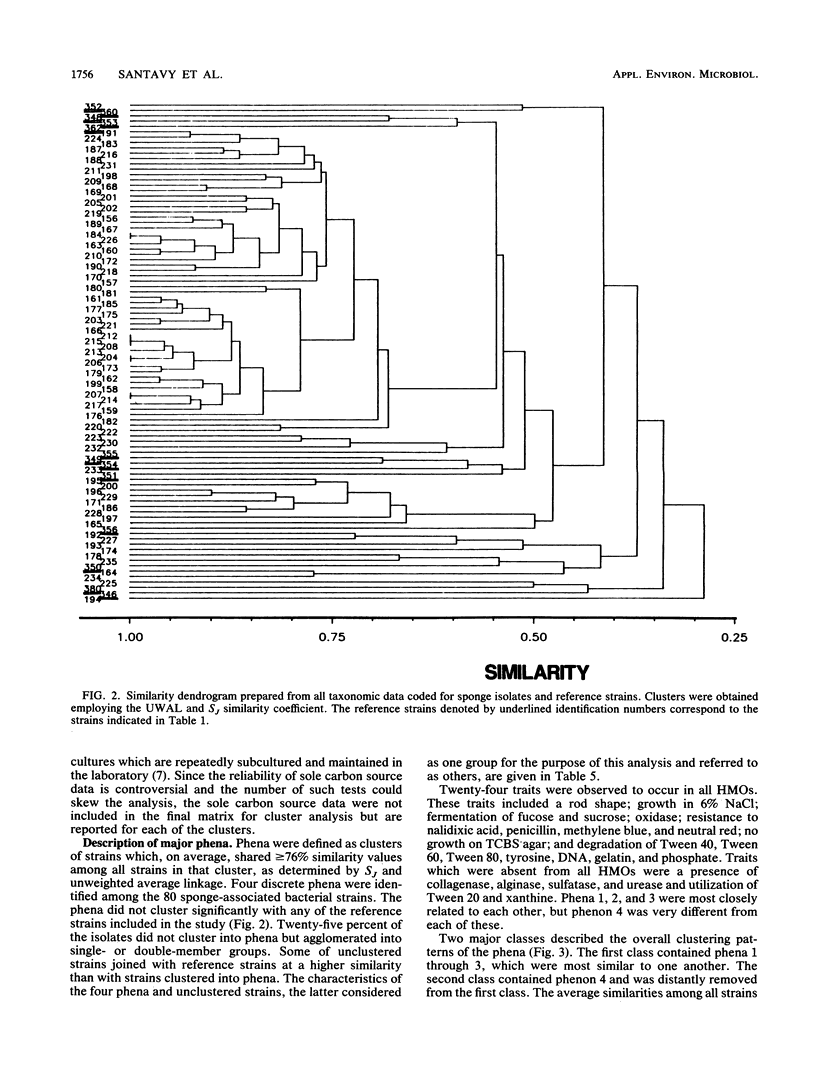
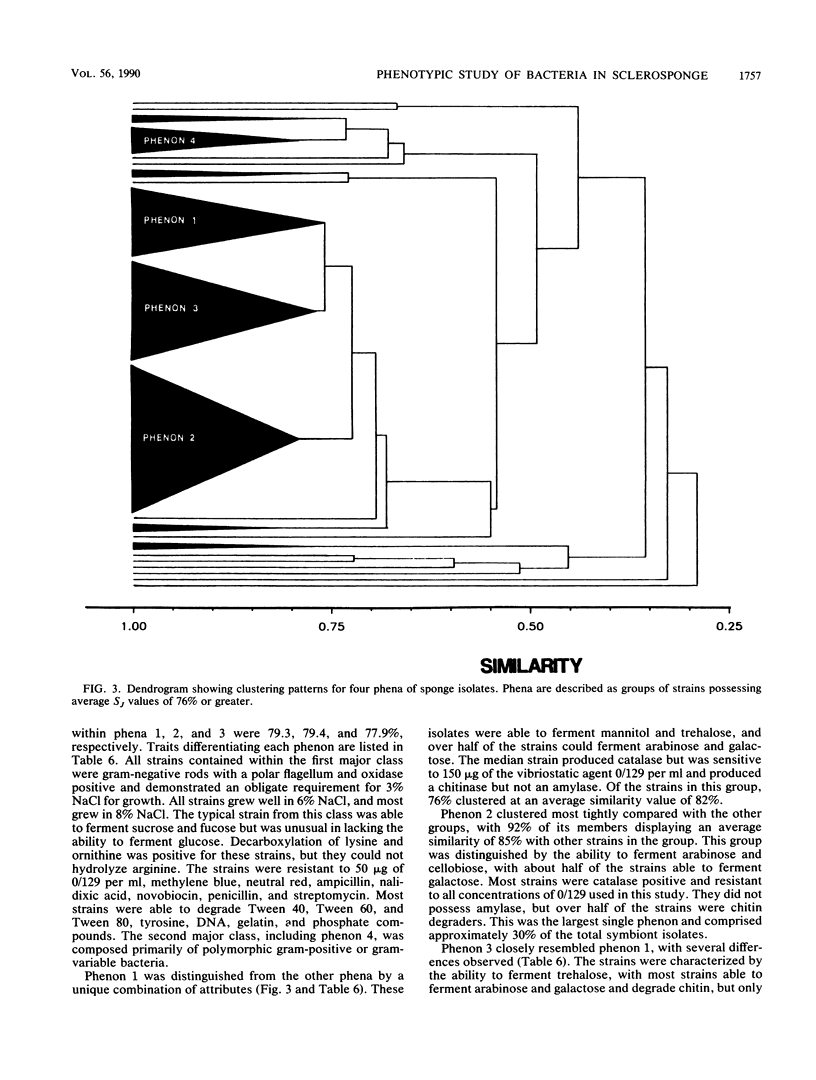
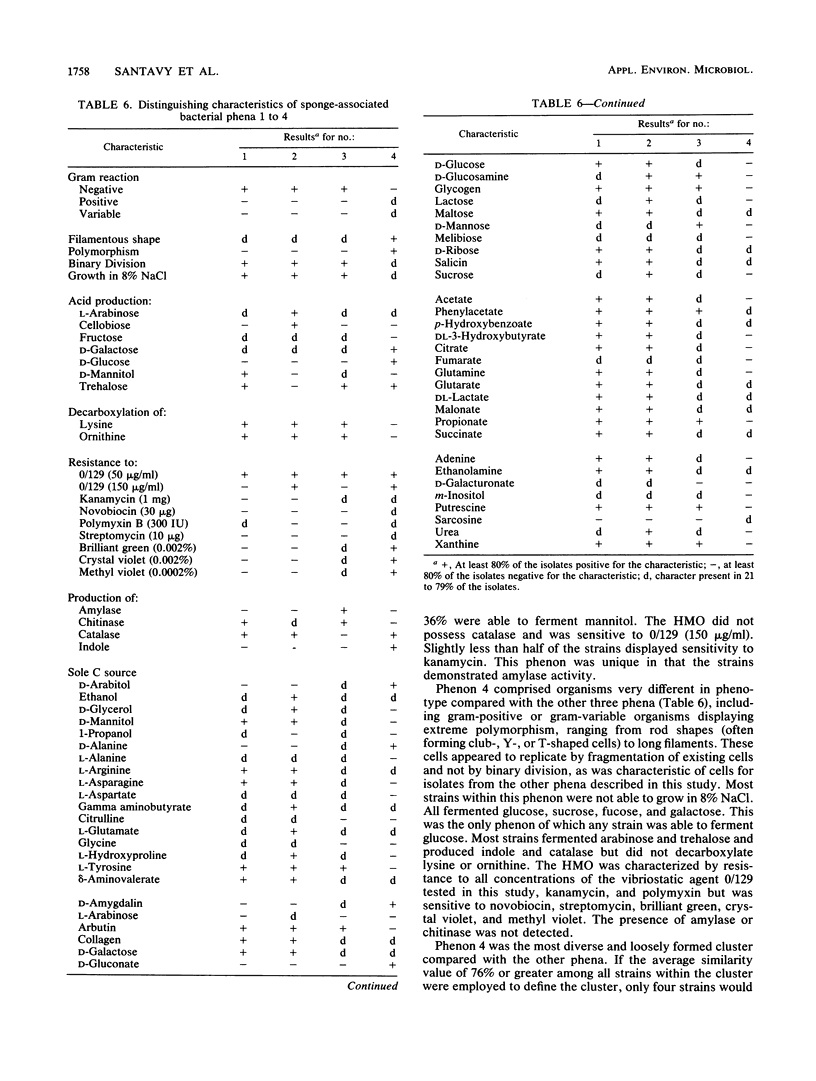
Abstract
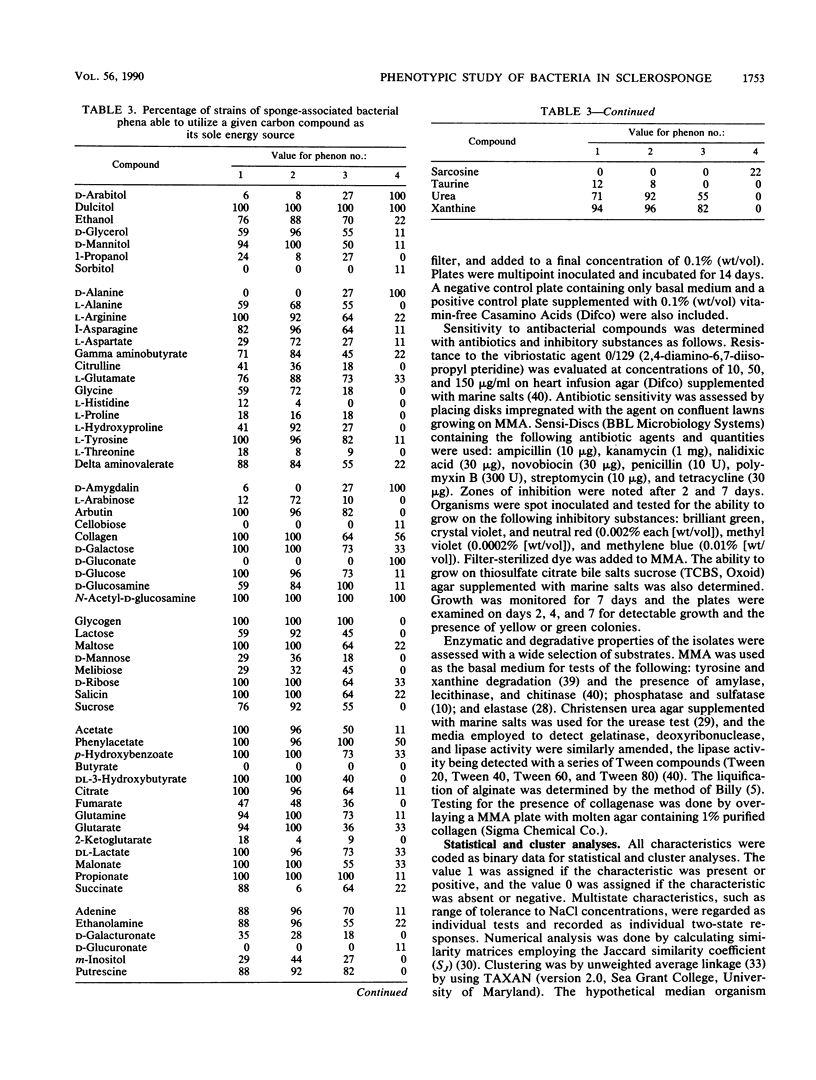
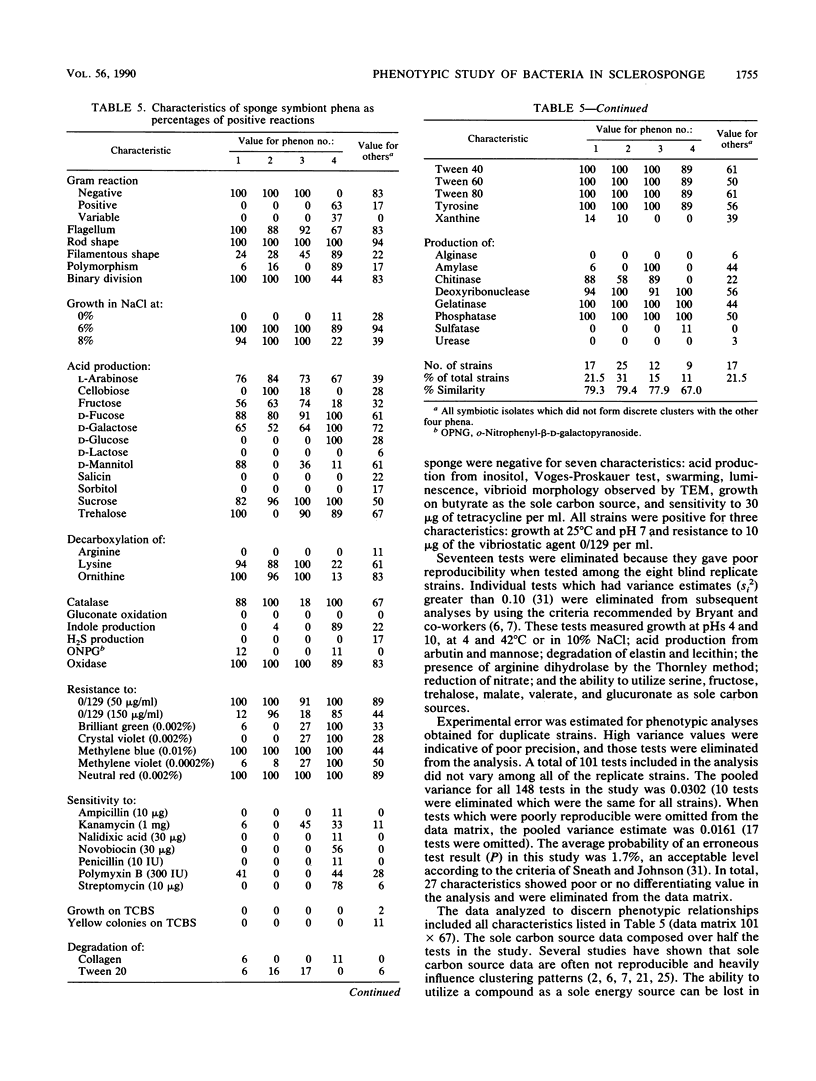
Heterotrophic bacteria associated with the Caribbean sclerosponge, Ceratoporella nicholsoni (Hickson), were found to occur extracellularly and were confined to the mesohyl regions of the sponge tissue. Physiological, metabolic, and morphological attributes of the culturable bacteria associated with the sponge were recorded by using numerical taxonomy methods for the analysis of 158 phenotypic attributes. Morphometric methods were used to determine the proportion of the total sponge-associated bacteria that were culturable by the methods employed, with the results ranging from 3 to 11% of the total bacteria inhabiting the sponge. Approximately 78% of the culturable bacteria clustered into four groups or phena, representing two previously undescribed Vibrio spp., an Aeromonas sp., and a coryneform- or actinomycete-like sp. Most of the bacteria were facultative anaerobes, fermenting sucrose and fucose but unusual in an inability to ferment glucose. This study was the first comprehensive study of heterotrophic bacteria associated with a sponge from the Caribbean basin, a region reputed to contain the most prolific sponge populations, with respect to biomass and diversity. The possible significance of these associations is discussed.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Austin B., Allen D. A., Zachary A., Belas M. R., Colwell R. R. Ecology and taxonomy of bacteria attaching to wood surfaces in a tropical harbor. Can J Microbiol. 1979 Apr;25(4):447–461. doi: 10.1139/m79-068. [DOI] [PubMed] [Google Scholar]
- Bertram J. F., Bolender R. P. Counting cells with stereology: random versus serial sectioning. J Electron Microsc Tech. 1990 Jan;14(1):32–38. doi: 10.1002/jemt.1060140106. [DOI] [PubMed] [Google Scholar]
- Billy C. Etude d'une bactérie alginolytique anaérobie: Clostridium alginolyticum n. sp. Ann Inst Pasteur (Paris) 1965 Jul;109(1):147–151. [PubMed] [Google Scholar]
- Bryant T. N., Lee J. V., West P. A., Colwell R. R. A probability matrix for the identification of species of Vibrio and related genera. J Appl Bacteriol. 1986 Nov;61(5):469–480. doi: 10.1111/j.1365-2672.1986.tb04309.x. [DOI] [PubMed] [Google Scholar]
- Bryant T. N., Lee J. V., West P. A., Colwell R. R. Numerical classification of species of Vibrio and related genera. J Appl Bacteriol. 1986 Nov;61(5):437–467. doi: 10.1111/j.1365-2672.1986.tb04308.x. [DOI] [PubMed] [Google Scholar]
- Buck J. D. Nonstaining (KOH) method for determination of gram reactions of marine bacteria. Appl Environ Microbiol. 1982 Oct;44(4):992–993. doi: 10.1128/aem.44.4.992-993.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feeherry F. E., Munsey D. T., Rowley D. B. Thermal inactivation and injury of Bacillus stearothermophilus spores. Appl Environ Microbiol. 1987 Feb;53(2):365–370. doi: 10.1128/aem.53.2.365-370.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glenville M., Broughton R., Wing A. M., Wilkinson R. T. Effects of sleep deprivation on short duration performance measures compared to the Wilkinson auditory vigilance task. Sleep. 1978 Winter;1(2):169–176. doi: 10.1093/sleep/1.2.169. [DOI] [PubMed] [Google Scholar]
- HUMPHREYS T. CHEMICAL DISSOLUTION AND IN VITRO RECONSTRUCTION OF SPONGE CELL ADHESIONS. I. ISOLATION AND FUNCTIONAL DEMONSTRATION OF THE COMPONENTS INVOLVED. Dev Biol. 1963 Aug;8:27–47. doi: 10.1016/0012-1606(63)90024-1. [DOI] [PubMed] [Google Scholar]
- Hammel I. Progression of errors in morphometry. Estimation of particle number density. J Histochem Cytochem. 1986 Jul;34(7):941–944. doi: 10.1177/34.7.3711652. [DOI] [PubMed] [Google Scholar]
- LISTON J., WIEBE W., COLWELL R. R. QUANTITATIVE APPROACH TO THE STUDY OF BACTERIAL SPECIES. J Bacteriol. 1963 May;85:1061–1070. doi: 10.1128/jb.85.5.1061-1070.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leifson E. Motile marine bacteria. IV. Ionic relationships of marine and terrestrial bacteria. Zentralbl Bakteriol Parasitenkd Infektionskr Hyg. 1970;125(2):170–206. [PubMed] [Google Scholar]
- Lemos M. L., Toranzo A. E., Barja J. L. Modified medium for the oxidation-fermentation test in the identification of marine bacteria. Appl Environ Microbiol. 1985 Jun;49(6):1541–1543. doi: 10.1128/aem.49.6.1541-1543.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maddocks J. L., Greenan M. J. A rapid method for identifying bacterial enzymes. J Clin Pathol. 1975 Aug;28(8):686–687. doi: 10.1136/jcp.28.8.686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popoff M., Véron M. A taxonomic study of the Aeromonas hydrophila-Aeromonas punctata group. J Gen Microbiol. 1976 May;94(1):11–22. doi: 10.1099/00221287-94-1-11. [DOI] [PubMed] [Google Scholar]
- SNEATH P. H. Some thoughts on bacterial classification. J Gen Microbiol. 1957 Aug;17(1):184–200. doi: 10.1099/00221287-17-1-184. [DOI] [PubMed] [Google Scholar]
- Sneath P. H., Johnson R. The influence on numerical taxonomic similarities of errors in microbiological tests. J Gen Microbiol. 1972 Sep;72(2):377–392. doi: 10.1099/00221287-72-2-377. [DOI] [PubMed] [Google Scholar]
- WEIBEL E. R., GOMEZ D. M. A principle for counting tissue structures on random sections. J Appl Physiol. 1962 Mar;17:343–348. doi: 10.1152/jappl.1962.17.2.343. [DOI] [PubMed] [Google Scholar]
- Wilkinson C. R. Interocean differences in size and nutrition of coral reef sponge populations. Science. 1987 Jun 26;236(4809):1654–1657. doi: 10.1126/science.236.4809.1654. [DOI] [PubMed] [Google Scholar]