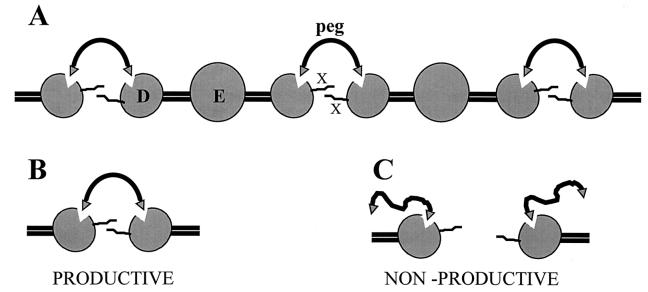
Figure 1.
The double-headed bis(Gly-Pro-Arg-Pro-amido)peg ligand is thought to promote the factor XIIIa-catalyzed crosslinking of fibrinogen or of its plasmin-derived D fragments by forming noncovalent complexes, illustrated by A and B. The central E and the distal D domains of the fibrinogen molecule are marked; the Gly-Pro-Arg-Pro heads of the ligand are represented by triangles (▿) and the 0,0′-bis(2-aminopropyl)polyethylene glycol tethers (denoted as peg) by solid lines. In the productive mode of binding into the polymerization pockets in the γ chains (triangular indentations in the D domains) the double-headed ligand brings together two D domains, apparently a prerequisite for optimal reaction for crosslinking (X) by factor XIIIa. Nonproductive mode of binding of the tethered ligand, when present in excess over the concentration of D in the medium, is illustrated in C.