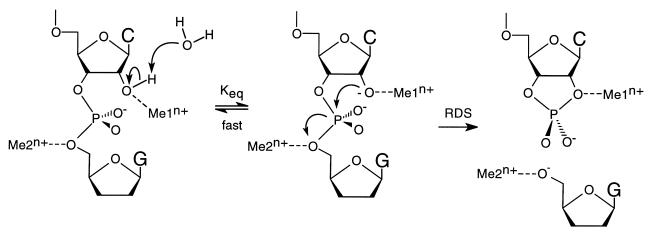
Figure 5.
Modified two-metal ion model for ribozyme cleavage. The metal ion in binding site 1 (Me1n+) coordinates directly to the 2′ oxygen, lowering the pKa of the 2′-OH proton. The 2′-OH proton is lost to the solvent in an equilibrium that is fast relative to the rate-determining cleavage step. The resulting 2′-alkoxide serves as the attacking nucleophile, displacing the 5′-oxygen of the leaving nucleotide (see arrows). The metal ion in binding site 2 (Me2n+) absorbs the developing charge on the leaving 5′-oxygen in the transition state of the rate-determining step. The nature of the metal ion coordination to the pro-R oxygen is not explicit in this model, although there is evidence for such an interaction. RDS, rate-determining step.