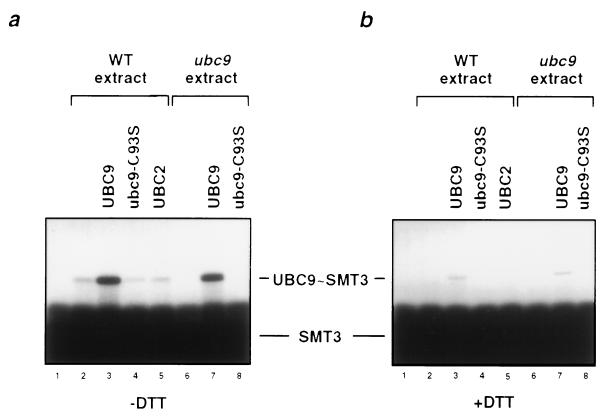
Figure 2.
Thioester complex formation between SMT3 and S. cerevisiae UBC9. Thioester reactions contained 32P-labeled SMT3, protein extracts from yeast WT (lanes 2–5) or from ubc9–1 mutant strain (lanes 6–8) (16), and crude extracts from bacteria expressing various E2 enzymes as indicated (lane 1 shows SMT3 only; lanes 2 and 6, without bacterially expressed E2 enzymes). The ubc9-C93S enzyme is a mutant of UBC9 in which the presumed catalytic-site cysteine residue at position 93 was altered to serine. Similar amounts of the respective E2 enzymes were used as determined by staining with Coomassie blue (data not shown). After 5 min at 25°C, reactions were stopped in the absence (a) or presence (b) of a reducing agent, and the products were subjected to SDS/PAGE followed by autoradiography. Positions of free 32P-labeled SMT3 and of the UBC9 thioester complex are indicated. Note that in the presence of protein extract derived from WT S. cerevisiae a band is detected (lane 2, 4, and 5) that comigrates with the SMT3-thioester complex of recombinant UBC9 (lane 3). Because this band is absent in the reactions with protein extracts derived from the ubc9–1 mutant (lane 6), this band is likely to represent the thioester adduct of SMT3 with endogenous UBC9 present in the WT strain extract.