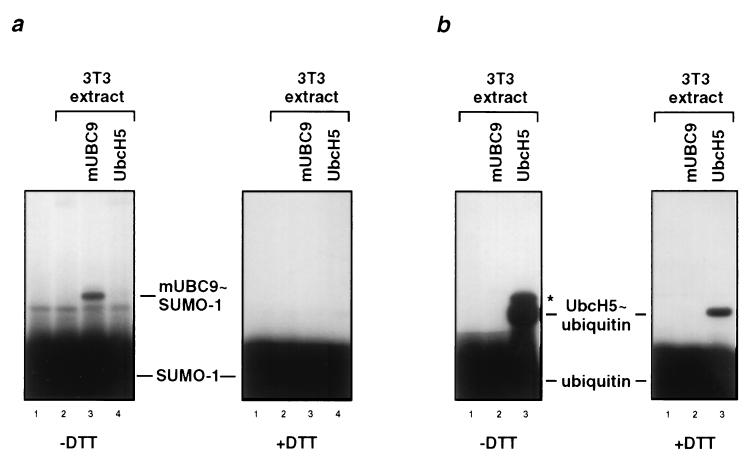
Figure 3.
Thioester complex formation of murine mUBC9 with SUMO-1 but not with ubiquitin. (a) 32P-labeled SUMO-1 was incubated with a Mono Q fraction derived from NIH 3T3 cell extracts and crude extracts from bacteria expressing mUBC9 (lane 3) or UbcH5 (lane 4) as indicated (lane 1 shows SUMO-1 only; lane 2 is without bacterially expressed E2 enzymes). Similar amounts of the respective E2 enzymes were used as determined by Coomassie blue staining (data not shown). After 5 min at 25°C, reactions were stopped in the absence or presence of DTT and subjected to SDS/PAGE followed by autoradiography. Positions of free 32P-labeled SUMO-1 and of the respective E2 thioester adducts are indicated. (b) Similar to a but 32P-labeled ubiquitin was incubated with NIH 3T3 cell extracts and extracts from bacteria expressing mUBC9 (lane 2) or UbcH5 (lane 3; lane 1 is without bacterially expressed E2 enzymes). The band marked by an asterisk presumably represents ubiquitin that is covalently linked to UbcH5 via an isopeptide bond. Note that the NIH 3T3 Mono Q fraction contained both the enzyme activities needed to activate ubiquitin and SUMO-1. However, the SUMO-1 activating enzyme activity is distinct from the well-characterized ubiquitin-activating enzyme (data not shown).