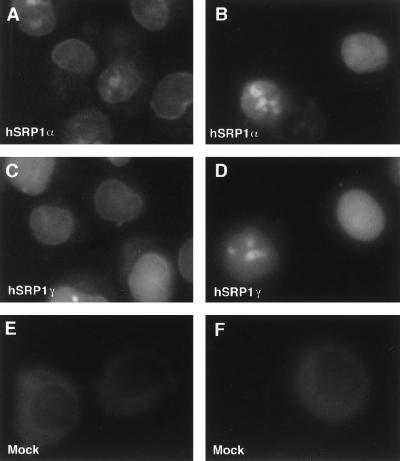
Figure 5.
In vitro nuclear protein import assays. Import of fluorescein-labeled BSA-NLS conjugate into digitonin-permeabilized HeLa cells was assayed in the presence of hSRP1α and importin β (A); hSRP1α, importin β, Ran, and p10/NTF2 (B); hSRP1γ and importin β (C); hSRP1γ, importin β, Ran, and p10/NTF2 (D); mock-purified extract and importin β (E); or mock-purified extract, importin β, Ran, and p10/NTF2 (F). The localization of the BSA-NLS substrate was analyzed by epifluorescence. Images were recorded by using a charge-coupled device camera. Quantitation of the uptake of BSA-NLS in B and D shows that hSRP1γ has only ∼30% of the import activity of hSRP1α. The protein amounts of hSRP1γ and α used in the assay were normalized with respect to their binding activity to importin β. Coomassie blue staining showed that roughly equal amounts of the two proteins were present in the transport assay (data not shown).