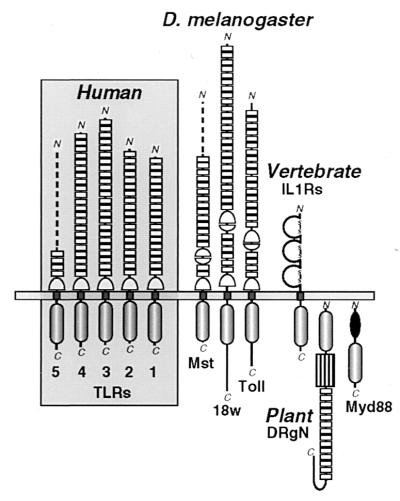
Figure 1.
Schematic comparison of the protein architectures of Drosophila and human TLRs and their relationship to vertebrate IL-1Rs and plant disease-resistance proteins. Three Drosophila (Dm) TLRs (Toll, 18w, and the Mst ORF fragment) (7, 11, 13, 28) are arrayed beside the four complete (TLRs 1–4) and one partial (TLR5) human (Hu) receptors. Individual LRRs in the receptor ectodomains that are flagged by PRINTS (19) are explicitely noted by boxes; top and bottom Cys-rich clusters that flank the C- or N-terminal ends of LRR arrays are respectively drawn by apposed half circles. The loss of the internal Cys-rich region in TLRs 1–5 largely accounts for their smaller ectodomains (558, 570, 690 and 652 aa, respectively) when compared with the 784- and 977-aa extensions of Toll and 18w. The incomplete chains of DmMst and HuTLR5 (519- and 153-aa ectodomains, respectively) are represented by dashed lines. The intracellular signaling module common to TLRs, IL-1Rs, the intracellular protein Myd88, and the tobacco disease-resistance gene N product (DRgN) is indicated below the membrane. Additional domains include the trio of Ig-like modules in IL-1Rs (disulfide-linked loops); the DRgN protein features a nucleotide-binding domain (box) and Myd88 has a death domain (solid oval).