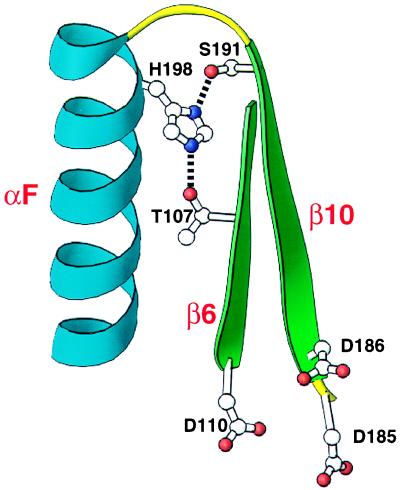
Figure 7.
T107/S191/H198 hydrogen bonding interaction. Potential hydrogen bonding interactions between T107 and H198 and between S191 and H198 are shown. The polypeptide backbone is shown for amino acid residues S105–D110 and D185–W212 from HIV-1 RT crystal structure 1har (14). Secondary structural elements shown are β6 (green), β10 (green), and αF (blue). Amino acid chains (T107, D110, D185, D186, S191, and H198) are shown. Side-chain nitrogen atoms are represented as purple dots, and side-chain oxygens as red dots. In the crystal structure 1har, the atomic distance between T107 Oγ1 and H198 Nɛ2 is 2.8 Å and between S191 Oγ and H198 Nδ1 is 2.7 Å. Side chains D110, D185, and D186 (catalytic aspartic acid triad of the active site) are included as a reference point. This structural representation is approximately inverted compared with the one in Fig. 5. This figure was generated by using the program ribbons (13).