Figure 4. Tbx20-specific siRNA transfected into primary chicken endocardial cushion cells results in decreased Tbx20 mRNA and protein expression.
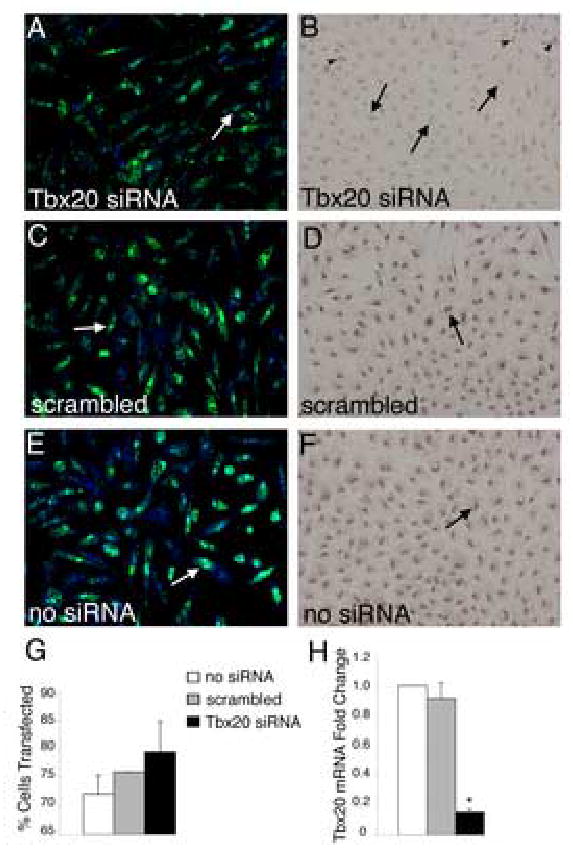
(A,C,E) Primary chicken endocardial cushion cells were co-transfected with siRNA and FITC-labeled fluorescent oligos (green) to visualize transfection efficiency. Cells were counterstained with TO-PRO-3 iodide (blue) to visualize the nuclei. Examples of positively transfected cells are indicated by white arrows. Transfection efficiency was determined as the number of cells with incorporation of FITC-labeled oligonucleotide/total TO-PRO-3 positive nuclei (G). These analyses demonstrated that cultures were consistently transfected at 70–80% efficiency. (B,D,F) Tbx20 protein expression was examined by immunohistochemistry with an antibody specific for Tbx20 in parallel cultures of siRNA-transfected cells. Cells transfected with Tbx20-specific siRNA (B) had significantly reduced nuclear staining (arrows indicate cells with reduced Tbx20 immunoreactivity) compared to controls (D,F, arrows indicate Tbx20 immunopositive cells). In Tbx20-specific siRNA transfected cultures (B), a subset of cells remained Tbx20 immunopositive (arrowheads). Tbx20 mRNA expression was quantified using real time RT-PCR (H). Cells transfected with Tbx20-specific siRNA had an 80% reduction in Tbx20 mRNA relative to untransfected and scrambled siRNA controls. Asterisks indicate statistically significant differences from control values and error bars represent standard error of the mean (n=3, P<0.01).
