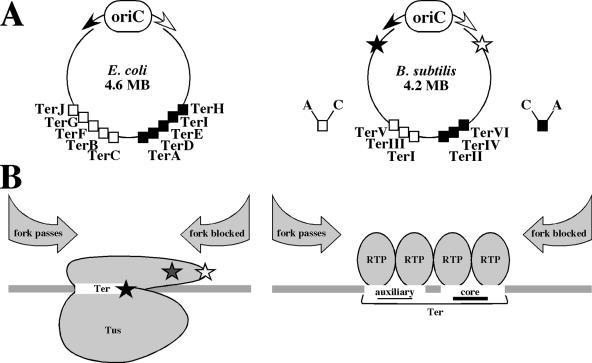
FIG. 1.
Termination of replication in E. coli and B. subtilis. (A) Organization of replication termini in E. coli (left) and B. subtilis (right). In circular bacterial chromosomes, two replication forks (white arrow, clockwise fork; black arrow, counterclockwise fork) start from the bidirectional origin of replication (oriC). Arrest sites are located at the terminus. In E. coli, TerC, TerB, TerF, TerG, and TerJ arrest the clockwise fork (white squares), and TerA, TerD, TerE, TerI, and TerH arrest the counterclockwise fork (black squares). In B. subtilis, TerI (also called IR I), TerIII, and TerV arrest the clockwise fork (white squares), and TerII (also called IR II), TerIV, and TerVI arrest the counterclockwise fork (black squares). Each Ter site contains core (C) and auxiliary (A) sites. TerI (IR I) is the most frequently used termination site. Checkpoint replication arrest sites in the B. subtilis chromosome are indicated by stars: the black star shows the position of arrest of the counterclockwise fork, and the white star shows the position of arrest of the clockwise fork. The positions of sites in both chromosomes are not to scale. (B) Replication termination proteins: Tus of E. coli (left) and RTP of B. subtilis (right) bound to the corresponding Ter sites. In the case of Tus (which binds Ter as a monomer), three models were proposed to explain the asymmetry of fork blocking. (i) The fork-blocking side of Tus contains protruding α helices (gray star), which protect the DNA binding domain from displacement. (ii) Certain amino acid residues on the fork-blocking side of Tus (white star) make protein-protein contacts with the helicase, blocking it. (iii) When the helicase approaches the Ter-Tus complex from the blocking side, it unwinds a part of the Ter site, exposing a particular cytosine residue (black star), which makes contact with Tus, leading to an increased stability of the Ter-Tus complex. In the case of RTP, the asymmetry of fork blocking comes from the different strengths of binding of the RTP dimers to the core and auxiliary sites. Within one Ter site, each core and auxiliary site binds a dimer of RTP (therefore, each Ter site binds four RTP monomers). If the core site is met by the fork first, the fork will be arrested, but if the auxiliary site is met first, both dimers will be displaced and the fork will proceed.