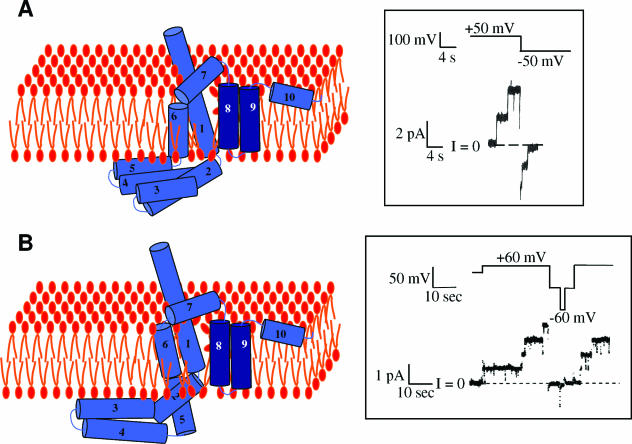
FIG. 18.
Schematic representations of the transmembrane segments of the open colicin channel. Only the channel-forming domain is shown. The depicted arrangement of the protein segments within the membrane derives from experiments, but the secondary structure (shown as blue, numbered, α-helical cylinders) follows that of the crystal structure and is purely speculative. Likewise, the distortion of the lipid bilayer is shown only as an acknowledgment of the potential involvement of lipids in the structure and not as a specific model. (A) Colicin Ia. Helices 2 to 5 are translocated across the membrane during channel opening. The inset shows the electrophysiological record of colicin Ia channels in a voltage-clamped lipid bilayer. Two channels open at positive voltage and close at negative voltage. (B) Colicin A. The colicin A channel resembles that of colicin Ia, but helices 2 and 5 are thought to be incompletely translocated. The inset shows colicin A channels in a lipid bilayer.