Figure 4.
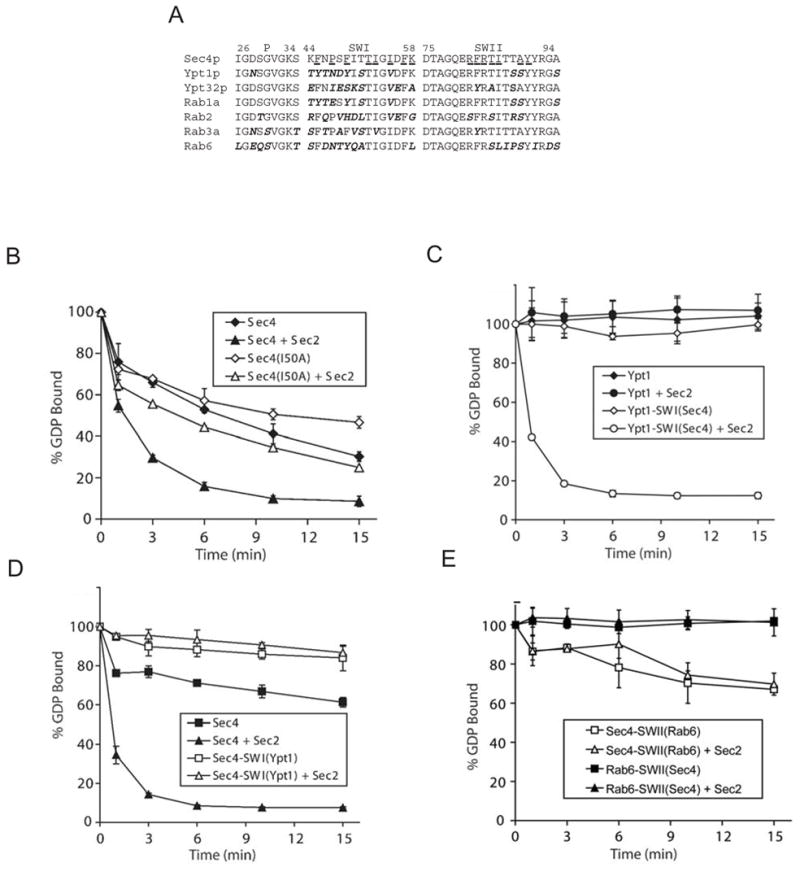
Importance of the Sec4p switch regions for GEF activity. (A) Sequences of the switch regions and P-loops of different Rabs. Sec4p residues within 4 Å of Sec2p in the complex are underlined. (B) Comparison of the release of [3H]GDP from 200 nM wild type Sec4p and from the Ile50Ala Sec4p mutant in the absence and presence of 100 nM Sec2p exchange domain. Sec2p catalyzed exchange is impaired for the mutant as compared to wild type Sec4p. Here Sec4p constructs are expressed as GST-fusion proteins. (C) Comparison of the release of [3H]GDP from 200 nM wild type Ypt1p and Ypt1p with switch I replaced by the Sec4p sequence, Ypt1-SWI(Sec4), in the absence or presence of 100 nM Sec2p exchange domain. While Sec2p does not act on Ypt1p, Ypt1-SWI(Sec4) is a substrate. (D) Comparison of the release of [3H]GDP from 200 nM wild type Sec4p and Sec4p with switch I replaced by the Ypt1p sequence, Sec4-SWI(Ypt1), in the absence or presence of 100 nM Sec2p exchange domain. Sec2p does not stimulate GDP exchange for Sec4-SWI(Ypt1). (E) Comparison of the release of [3H]GDP from 200 nM Sec4p with switch II replaced by the Rab6 sequence, Sec4p-SWII(Rab6), and Rab6 with a Sec4p switch Rab6-SWII(Sec4), in the absence or presence of 100 nM Sec2p exchange domain. Sec2p does not act on Sec4p-SWII(Rab6) or Rab6-SWII(Sec4). Sec4p and Rab6 constructs are expressed as GST-fusions. Error bars represent standard deviation.
