Figure 4.
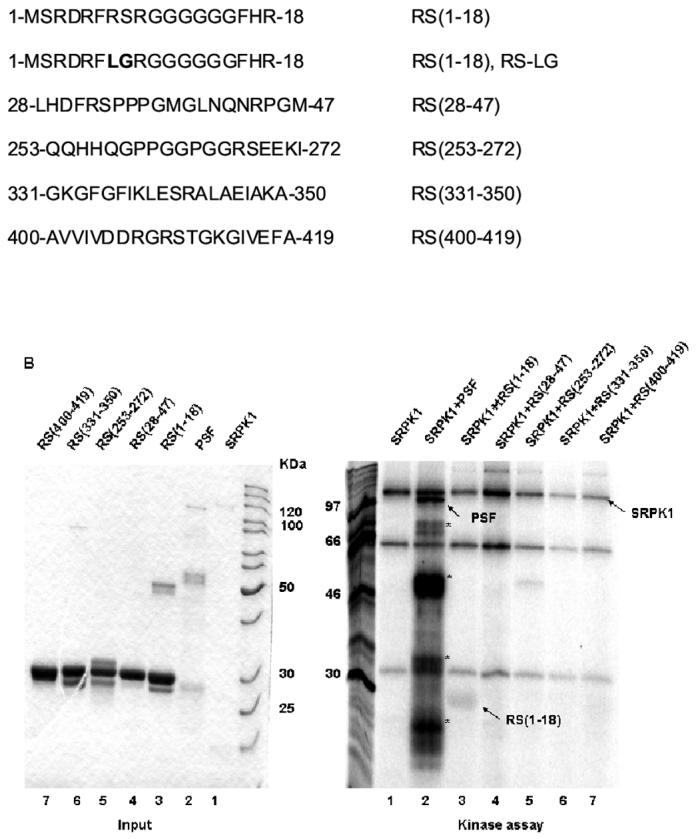
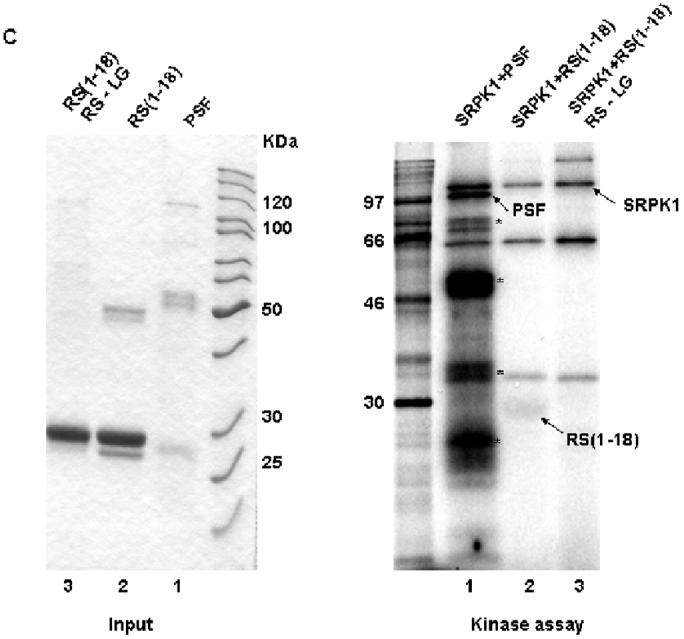
An N-terminal RS dipeptide within PSF is phosphorylated by SRPK1. (A). Amino acid sequences spanning the five RS dipeptides, the two mutations of 7-RS-8 within the N-terminal (1-18) region, and their relative positions within PSF. All peptides were expressed and purified as GST fusion proteins. (B) The indicated bacterially expressed GST fusion proteins were incubated with SRPK1 in the presence of [γ-32P]ATP at 23°C for 30 min (right panel). Integrity and equal inputs for each fusion protein was confirmed by Commassie staining (left panel). (C) Bacterially expressed RS(1-18) wildtype and mutated peptides (left panel) were incubated with SRPK1 in the presence of [γ-32P]ATP at 23°C for 30 min (right panel). The samples were resolved on 10% SDS-PAGE and visualized by phosphoimaging. Asterisks denote degradation products of full-length PSF. The apparent increase in signal of SRPK1-mediated phosphorylation in this experiment relative to that in Fig. 3B results from the use of a higher specific activity 32P-ATP substrate
