Figure 3.
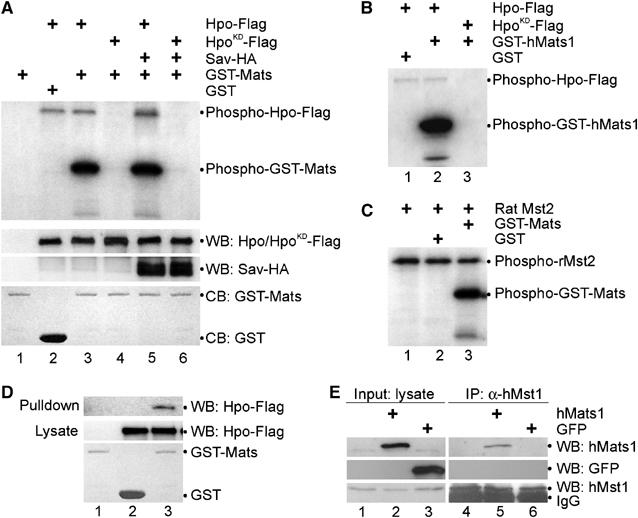
Mats associates with Hpo and is phosphorylated by Hpo protein kinase. (A) Hpo specifically phosphorylates Mats in vitro. pAc-hpo-Flag, pAc-hpoKD-Flag and pAc-sav-HA were transfected into S2 cells as indicated. Lysates of transfected cells were used for Western blot (WB) to show the inputs (middle two panels). Anti-Flag antibody was used to immunoprecipitate (IP) Hpo-Flag or HpoKD-Flag proteins for an in vitro kinase assay, in which GST-Mats or GST proteins were used as substrates (the bottom panel; CB: Coomassie blue staining). Hpo autophosphorylation and Mats phosphorylation by Hpo/HpoKD are shown in the top panel. (B) GST was not a substrate of Hpo kinase (lane 1). Hpo (lane 2) but not HpoKD (lane 3) can phosphorylate human Mats1 in vitro. (C) A rat Hpo ortholog, Mst2 kinase, can phosphorylate GST-Mats (lane 3) but not GST (lane 2) in vitro. Like Hpo, Mst2 mediates autophosphorylation (lanes 1–3). (D) In an in vitro pull-down assay, Mats was shown to associate with Hpo. Bacterially produced GST-Mats (lanes 1 and 3) or GST (lane 2) proteins were tested for their ability to interact with Hpo-Flag (lanes 2 and 3) produced in transfected S2 cells. (E) Human Mats1 was co-immunoprecipitated with the endogenous human Mst1. pCMV-hMats1 and pCMV-GFP were transfected into HEK293T cells as indicated. Inputs of hMats1 (lane 2), GFP (lane 3) and hMst1 (lanes 1–3) proteins are shown in panels on the left. Immunoprecipitations were carried out using anti-hMst1 antibodies to isolate the endogenous hMst1 protein (lanes 4-6, the bottom-right panel), and hMats1 (lane 5) but not GFP (lane 6) was co-immunoiprecipitated (top two panels on the right).
