Figure 2.
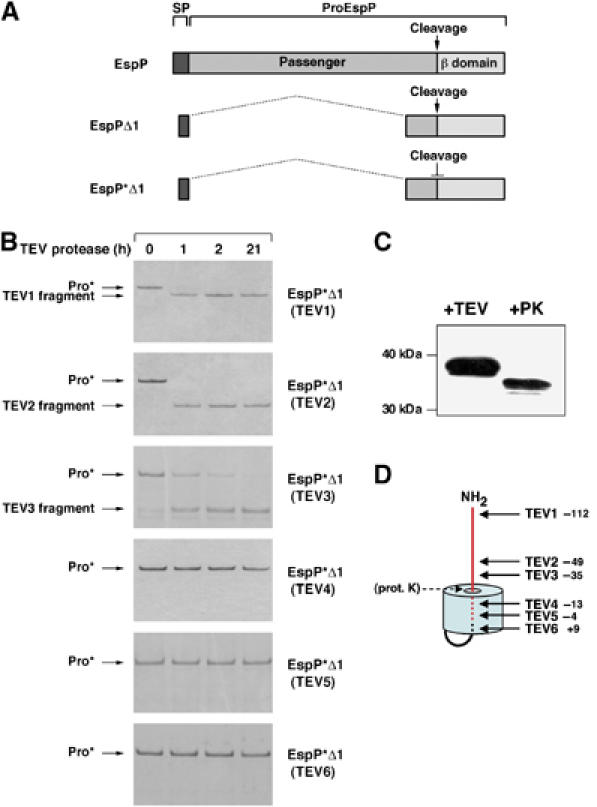
The EspP passenger domain cleavage site is embedded within the β barrel. (A) Illustration of EspP, EspPΔ1 and EspP*Δ1, a previously described non-cleavable version of EspPΔ1 that harbors the double mutation N1023S/N1024S. ProEspP is the form of the protein that contains covalently linked passenger and β domains but no signal peptide (SP). (B) Derivatives of EspP*Δ1 that contain a TEV protease site were purified and incubated with TEV protease for various lengths of time. Reaction products were then analyzed by SDS–PAGE on 4–12% NuPAGE gels using MES buffer (Invitrogen). (C) Purified EspP*Δ1(TEV3) was incubated with TEV protease or proteinase K (PK). The major cleavage product was detected by Western blot using an antiserum directed against an EspP C-terminal peptide. (D) Model of the topology of EspP*Δ1 derived from the data shown in (B) and (C). The distance of each TEV insertion from the passenger domain cleavage site is indicated. The passenger domain is shown in red.
