Figure 4.
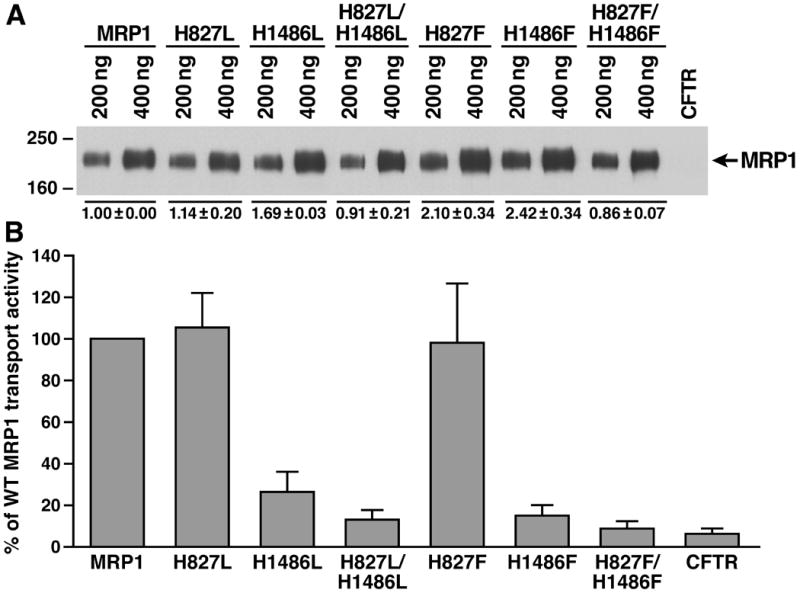
Prevention of hydrogen bond formation of the residue at 1486 in NBD2 with the γ -phosphate of the bound ATP and the putative catalytic base in this site significantly decreased the ATP-dependent LTC4 transport activity. A. Expression of wild-type and variant mutants (shown on top of the gel) of full length human MRP1 proteins in BHK cells at 37 ºC. Membrane vesicles were prepared from BHK cells expressing either wild-type or variant mutants of human MRP1. The amounts of membrane vesicle protein loaded are indicated on the top of the gel. Molecular weight markers are indicated on the left. MRP1 on the right indicates the 190 kDa complex-glycosylated human MRP1 proteins that were detected in western blot by employing the monoclonal antibody 42.4. The intensities of the 190 kDa bands were determined by a scanning densitometer. The mean ratios (n = 3), considering the amount of wild type MRP1 as 1, of the mutant proteins are listed at the bottom of the gel. B. Relative rate of ATP-dependent LTC4 transport activity. The assays were carried out in a 30 μ l solution containing 3 μ g of membrane vesicles (the amount of MRP1 protein determined in A was adjusted to a similar amount by adding varying amount of membrane vesicles prepared from BHK cells transfected with human CFTR cDNA and 4 mM AMP (used as a control) or 4 mM ATP at 37 °C for 4 minutes. The amount of LTC4 bound to the membrane vesicles in the presence of 4 mM AMP was subtracted from the corresponding samples in the presence of 4 mM ATP. The data are means ± S.D. of four triplicate-determinations.
