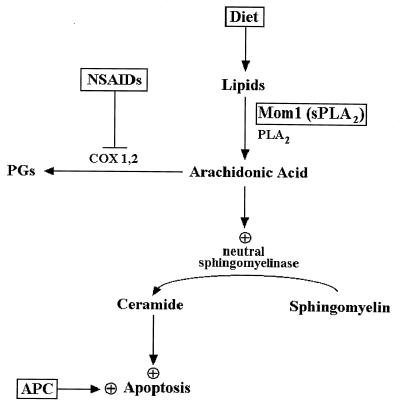
Figure 5.
Model relating AA and ceramide to colorectal cancer chemoprevention. Mutation of the APC gene initiates colorectal tumorigenesis and results in abnormally decreased apoptosis (16). Perturbation of lipid metabolism by pharmacological, dietary, or genetic means can partially correct the deficits. AA is generated by cytosolic and secreted phospholipase A2, which hydrolyzes plasma membrane lipids or lipids derived from the diet. AA is normally used as a substrate by the COXs to produce eicosanoids such as prostaglandins. NSAIDs inhibit the activity of the COXs, which increases the cellular pool of AA. AA stimulates neutral sphingomyelinase activity (18, 37), which catalyzes the hydrolysis of sphingomyelin to generate ceramide. Ceramide acts as second messenger that activates the cellular apoptotic machinery. The Mom1 gene is a modifier of polyp formation in the Min mouse, a model for APC mutation-induced colon tumors. The Mom1 gene encodes a secreted phospholipase A2, an enzyme predicted to increase the level of free AA. Inactivating mutations in Mom1 predispose Min mice to developing intestinal polyps and would be expected to result in reduced levels of AA (34–36). This in turn could result in decreased production of ceramide and therefore a relative resistance to programmed cell death and increased tumor susceptibility. The lipid compositions of diets are known to affect colon cancer risk (41, 42). Diets rich in unsaturated fatty acids such as AA are associated with a decreased incidence of colon cancer (43). This effect could be due to increased levels of AA and subsequent increased susceptibility to apoptosis as described above.