Abstract
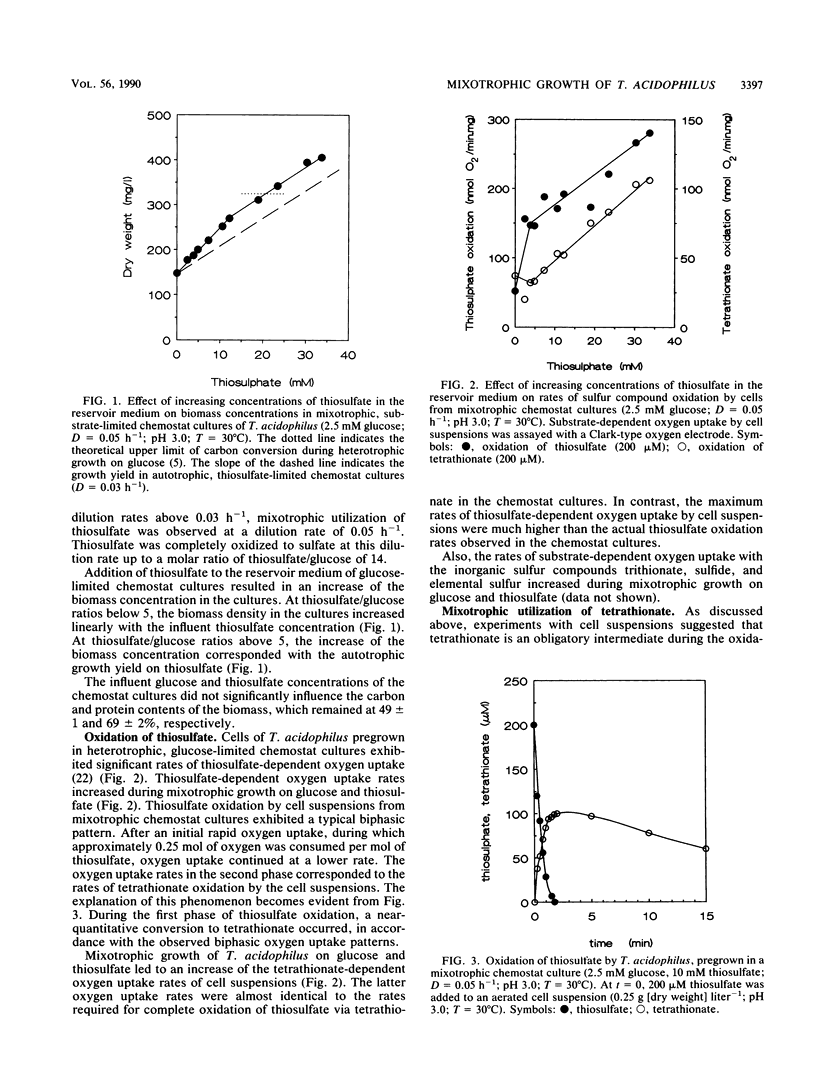
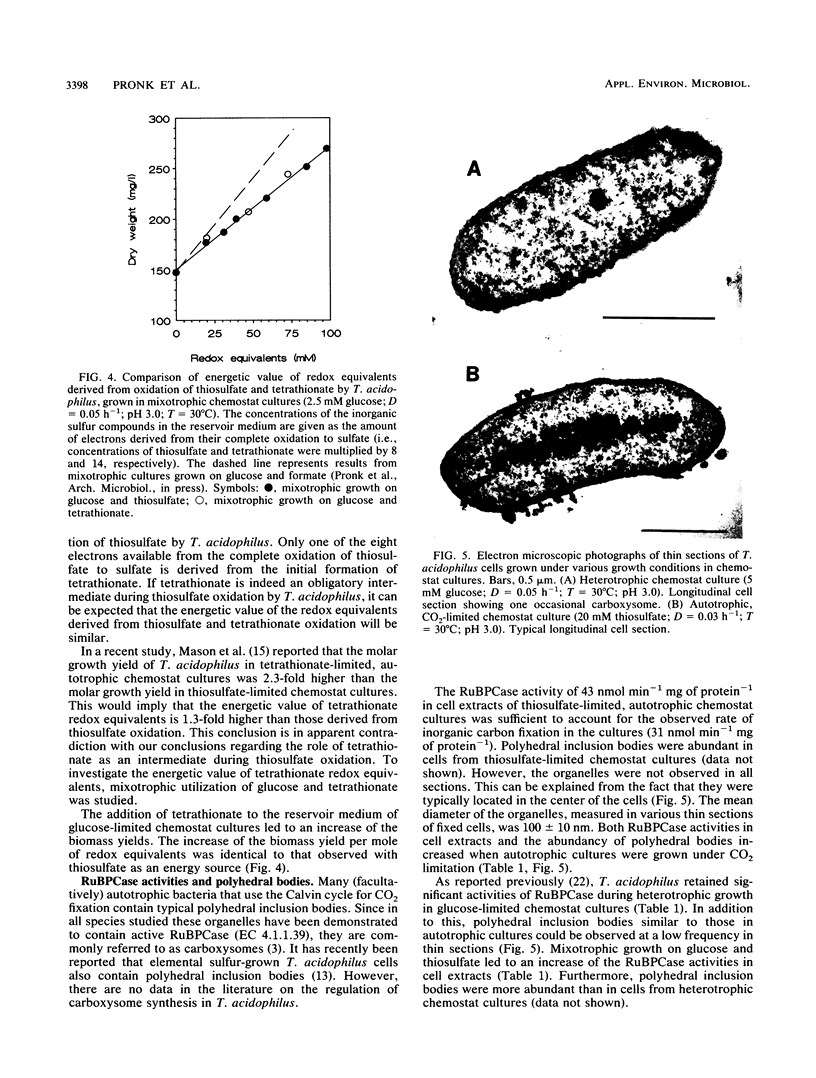
Mixotrophic growth of the facultatively autotrophic acidophile Thiobacillus acidophilus on mixtures of glucose and thiosulfate or tetrathionate was studied in substrate-limited chemostat cultures. Growth yields in mixotrophic cultures were higher than the sum of the heterotrophic and autotrophic growth yields. Pulse experiments with thiosulfate indicated that tetrathionate is an intermediate during thiosulfate oxidation by cell suspensions of T. acidophilus. From mixotrophic growth studies, the energetic value of thiosulfate and tetrathionate redox equivalents was estimated to be 50% of that of redox equivalents derived from glucose oxidation. Ribulose 1,5-bisphosphate carboxylase (RuBPCase) activities in cell extracts and rates of sulfur compound oxidation by cell suspensions increased with increasing thiosulfate/glucose ratios in the influent medium of the mixotrophic cultures. Significant RuBPCase and sulfur compound-oxidizing activities were detected in heterotrophically grown T. acidophilus. Polyhedral inclusion bodies (carboxysomes) could be observed at low frequencies in thin sections of cells grown in heterotrophic, glucose-limited chemostat cultures. Highest RuBPCase activities and carboxysome abundancy were observed in cells from autotrophic, CO2-limited chemostat cultures. The maximum growth rate at which thiosulfate was still completely oxidized was increased when glucose was utilized simultaneously. This, together with the fact that even during heterotrophic growth the organism exhibited significant activities of enzymes involved in autotrophic metabolism, indicates that T. acidophilus is well adapted to a mixotrophic lifestyle. In this respect, T. acidophilus may have a competitive advantage over autotrophic acidophiles with respect to the sulfur compound oxidation in environments in which organic compounds are present.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Codd G. A. Carboxysomes and ribulose bisphosphate carboxylase/oxygenase. Adv Microb Physiol. 1988;29:115–164. doi: 10.1016/s0065-2911(08)60347-1. [DOI] [PubMed] [Google Scholar]
- Guay R., Silver M. Thiobacillus acidophilus sp. nov.; isolation and some physiological characteristics. Can J Microbiol. 1975 Mar;21(3):281–288. doi: 10.1139/m75-040. [DOI] [PubMed] [Google Scholar]
- Handley P. S., Hargreaves J., Harty D. W. Ruthenium red staining reveals surface fibrils and a layer external to the cell wall in Streptococcus salivarius HB and adhesion deficient mutants. J Gen Microbiol. 1988 Dec;134(12):3165–3172. doi: 10.1099/00221287-134-12-3165. [DOI] [PubMed] [Google Scholar]
- Harrison A. P., Jr The acidophilic thiobacilli and other acidophilic bacteria that share their habitat. Annu Rev Microbiol. 1984;38:265–292. doi: 10.1146/annurev.mi.38.100184.001405. [DOI] [PubMed] [Google Scholar]
- Matin A., Wilson B., Zychlinsky E., Matin M. Proton motive force and the physiological basis of delta pH maintenance in thiobacillus acidophilus. J Bacteriol. 1982 May;150(2):582–591. doi: 10.1128/jb.150.2.582-591.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muyzer G., de Bruyn A. C., Schmedding D. J., Bos P., Westbroek P., Kuenen G. J. A Combined Immunofluorescence-DNA-Fluorescence Staining Technique for Enumeration of Thiobacillus ferrooxidans in a Population of Acidophilic Bacteria. Appl Environ Microbiol. 1987 Apr;53(4):660–664. doi: 10.1128/aem.53.4.660-664.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purohit K., McFadden B. A., Shaykh M. M. D-Ribulose-1,5-bisphosphate carboxylase and polyhedral inclusion bodies in Thiobacillus intermedius. J Bacteriol. 1976 Jul;127(1):516–522. doi: 10.1128/jb.127.1.516-522.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SORBO B. A colorimetric method for the determination of thiosulfate. Biochim Biophys Acta. 1957 Feb;23(2):412–416. doi: 10.1016/0006-3002(57)90346-3. [DOI] [PubMed] [Google Scholar]
- Sinha D. B., Walden C. C. Formation of polythionates and their interrelationships during oxidation of thiosulfate by Thiobacillus ferrooxidans. Can J Microbiol. 1966 Oct;12(5):1041–1054. doi: 10.1139/m66-139. [DOI] [PubMed] [Google Scholar]
- Zychlinsky E., Matin A. Cytoplasmic pH homeostasis in an acidophilic bacterium, Thiobacillus acidophilus. J Bacteriol. 1983 Dec;156(3):1352–1355. doi: 10.1128/jb.156.3.1352-1355.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zychlinsky E., Matin A. Effect of starvation on cytoplasmic pH, proton motive force, and viability of an acidophilic bacterium, Thiobacillus acidophilus. J Bacteriol. 1983 Jan;153(1):371–374. doi: 10.1128/jb.153.1.371-374.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]



