Figure 3.
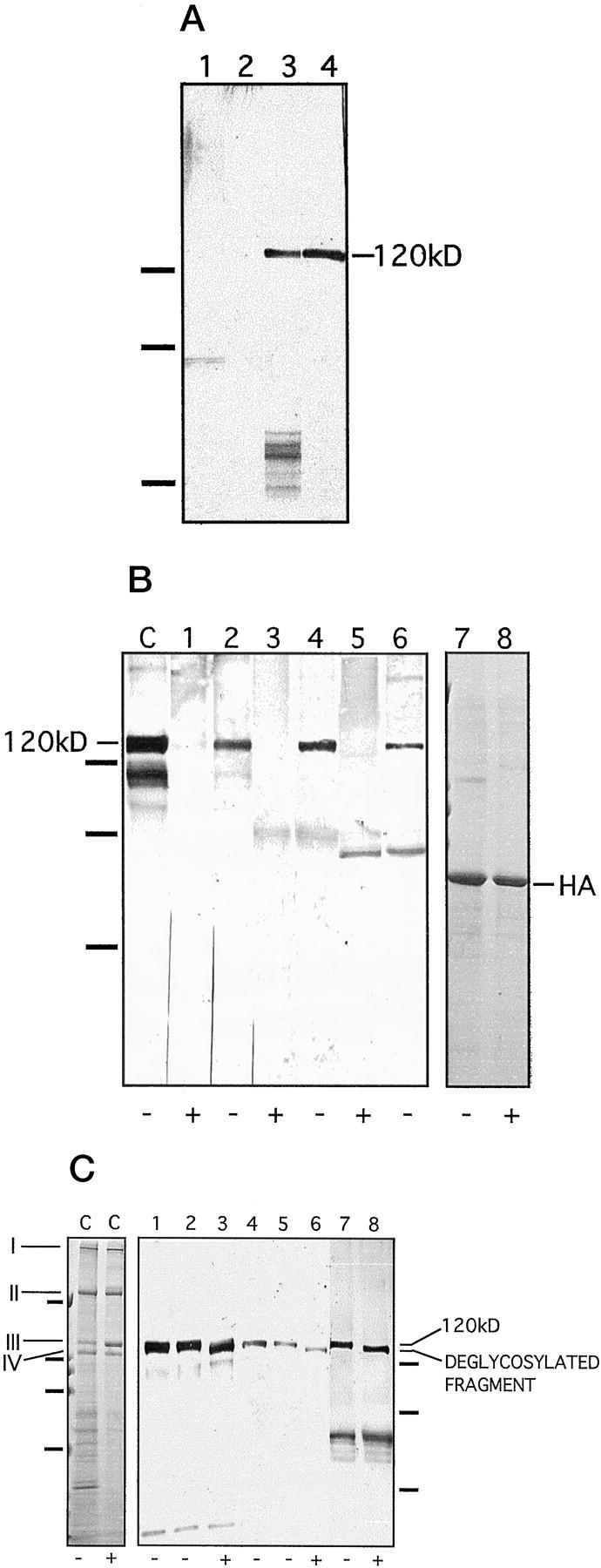
Characterization of the soluble ectodomain as an antigen. A: The soluble ectodomain was immunoprecipitated from keratinocyte medium with the NC16a antibody and cross-blotted with patient sera. Lane 1: Medium of mutant, collagen XVII-deficient keratinocytes, blotted with IgG antibodies from a pemphigoid serum. Lane 2: Medium of mutant, collagen XVII-deficient keratinocytes, blotted with IgA antibodies form an LAD serum. Lane 3: Normal keratinocyte medium, blotted with a LAD serum. Lane 4: Normal keratinocyte medium, blotted with a pemphigoid serum. The absence of the 120-kd band in the samples from collagen XVII-deficient keratinocyte cultures verifies its identity as the soluble ectodomain. On the left, migration positions of molecular weight markers are indicated. From top to bottom: 112, 80, and 50 kd. B: Immunoblotting of the soluble ectodomain treated with (lanes 1, 3, and 5) or without (lanes 2, 4, and 6) highly purified bacterial collagenase under identical conditions. The collagenase digestion abolished the 120-kd band detected with the NC16a antibody (lanes 1 and 2), with pemphigoid serum 1803/95 (lanes 3 and 4), and with a LAD serum 44/98 (lanes 5 and 6). C: an unincubated, concentrated ectodomain control. In this lane, the lower bands of approximately 105 and 90 kd represent degradation products of the shed ectodomain, as described previously. 1 As a control for nonspecific proteolytic activity in the collagenase preparation, human albumin (HA) was incubated without (lane 7) or with (lane 8) collagenase conditions identical to those as above. The enzyme treatment did not affect the protein, proving the specificity of the collagenase and excluding other proteolytic activities. Lanes 7 and 8 were stained with Coomassie blue. +, with enzyme; −, without enzyme. On the left, migration positions of molecular mass markers are indicated. From top to bottom: 112, 80, and 50 kd. C: Deglycosylation with N-glycosidase F. As a control, lane C on the left shows deglycosylation of rat-tail tendon collagen I under conditions identical to those used for collagen XVII. As expected, treatment with N-glycosidase F did not result in a shift in the mobility of the γ (I), β (II), α1 (III), or α2 (IV) chains of collagen I, a protein that does not contain N-linked carbohydrates. This experiment also showed that the enzyme preparation did not have contaminating proteolytic activity. Lane C is stained with Coomassie blue. On the left, the positions of the molecular mass markers are indicated for this gel. From top to bottom: 200, 112, 80, and 50 kd. Lanes 1–8 show an immunoblot of deglycosylated soluble ectodomain of collagen XVII. The ectodomain was incubated without (lanes 2, 5, and 7) and with (lanes 3, 6, and 8) N-glycosidase F. Lanes 1 and 4 show unincubated controls. Lanes 1–3 were detected with the control antibody Ecto1, lanes 4–6 with LAD serum 39/98, and lanes 7 and 8 with the LAD serum 61/98. N-Glycosidase F digestion resulted in a shift in the mobility of the protein but did not eliminate the reactivity with the IgA autoantibodies. We have shown previously 1 that IgG antibodies recognize the ectodomain after deglycosylation. +, with enzyme; −, without enzyme. On the right, migration positions of molecular mass markers are indicated for the immunoblot. From top to bottom: 112, 80, and 50 kd.
