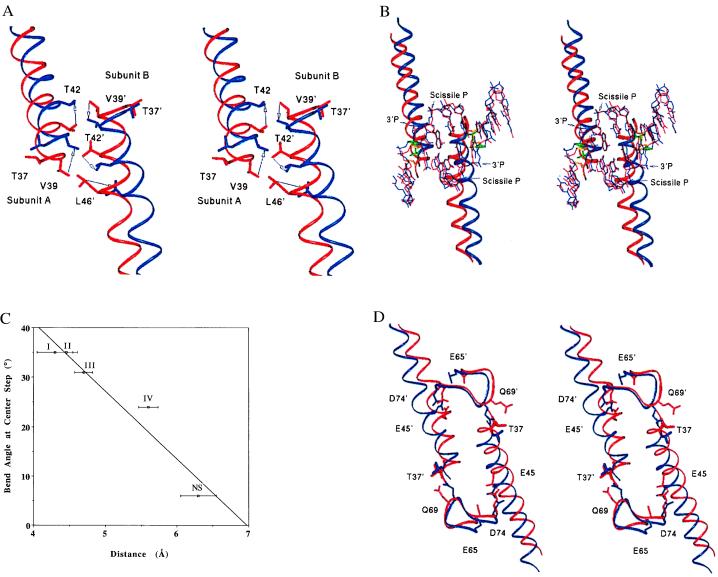
Figure 3.
(A) Superposition (based on the R-loop residues 184–187) of the least-bent form IV (red) and the most-bent form I (blue), showing interdigitation of Leu-46, Thr-42, and Val-39 at the B-helix interface. Arrows indicate the antiparallel movements of the helices during the 11° bending of the DNA in progressing from form IV to form I. Distances between Cα T42 (blue) and Cα Thr-42 (red) are 3.4 Å in subunit A and 2.2 Å in subunit B. (B) Propagation of the B-helix conformational changes to DNA bending, with structures superimposed as in A. The B-helices and DNA from crystal form I (blue) and crystal form IV (red) are shown, with coupling between the Thr-37–Thy-8 ribose contact and the B-helix translation also illustrated. Thr-37 and the ribose sugar in form I are shown in green in thicker bonds for clarity. These groups in form IV are shown in gold. (C) Plot of the center-step DNA-bending angle as a function of distance between Thr-37Cβ and Thy-8-C4′. Error bars for crystal forms II and IV reported here indicate coordinate error as calculated with the program sigmaa. The error bars for crystal forms I and III (9, 10) were estimated from the resolutions of the data sets, based on calculations from truncated penicillopepsin data, and may represent underestimates (33). The points represent the average Thr-37(Cβ)-ribose(C4′) distance for the two monomer subunits of each dimer. Roman numerals adjacent to the data points indicate the crystal form (Tables 1 and 3). (D) Propagation of the B-helix conformational change into the adjacent Q-loops. Residues in the active sites Glu-45, Glu-65, and Asp-74 are shown, as is the Thr-37–Gln-69 contact in crystal form I (blue) and crystal form IV (red). The superposition was done as in A.