Fig. 1.
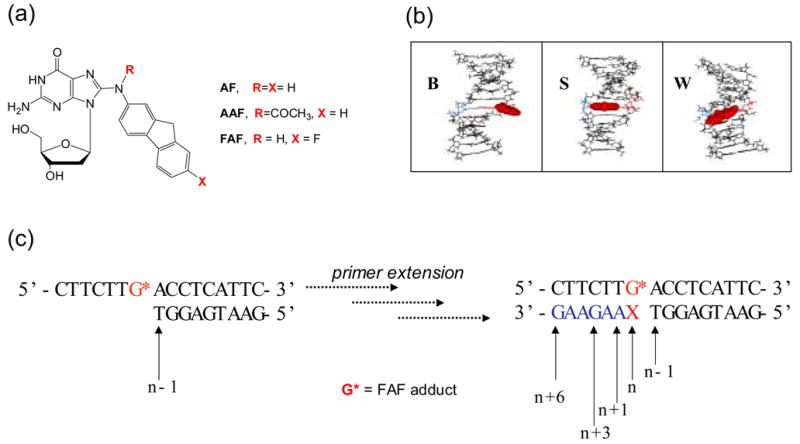
(a) Structures of C8-substituted dG-aminofluorene adducts. (b) Views from the minor-groove of a duplex for the three conformational motifs of the AF-modified DNA: B-, S-, and W-conformers. The modified dG and the complementary base (dC for B and S; dA for W) residues are shown in red and blue lines, respectively and the AF-moiety is highlighted with red CPK. In B conformer, anti-[AF]dG maintains Watson-Crick hydrogen bonds, thereby placing the aminofluorene ring in the major groove with displacement of the modified dG and partner dC. The aminofluorene moiety of the S-conformer stacks into the helix with the modified dG in the syn-glycosidyl conformation (base-displaced). The modified dG of the W-conformer adopts syn-configuration; however, the steric constraint at the lesion site allows the aminofluorene ring wedged into the surface of the narrow minor groove. (c) Sequence context studied (G* = FAF-adduct; X = C, FAF-G:C match series; X = A, FAF-G:A mismacth series).
