Fig. 2.
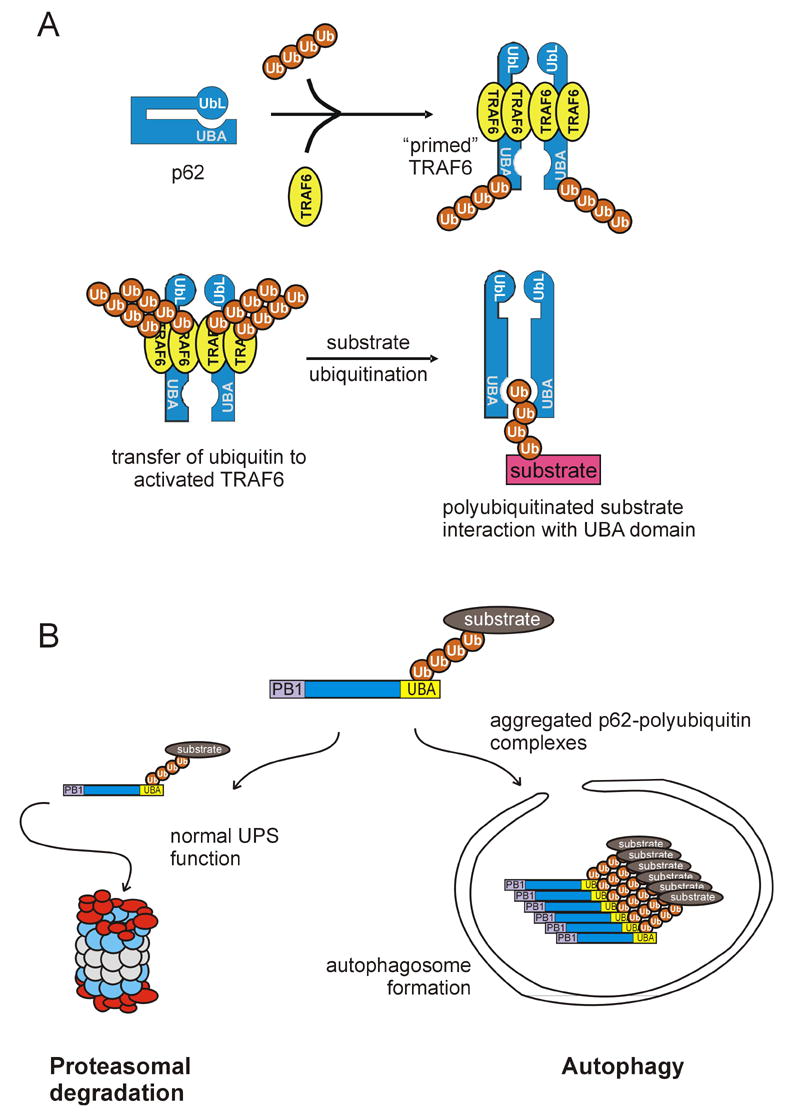
Models of p62 function. a) p62 can act as a scaffold for the K63 polyubiquitination of TRAF6 specific substrates. In a native form, the UbL domain of p62 may interact with its UBA domain, prior to interaction with ubiquitin. Next, the protein may “open” allowing access to multiple protein binding domains. Following p62 dimerization through the PB1 domain, dimerized TRAF6 binds to p62 at the TRAF6-binding site allowing the transfer of K63 polyubiquitin chains generated from TRAF6 to specific substrates. K63-tagged substrates can interact with the UBA domain of p62. b) p62 may reside at the intersection of two degradation pathways. Once the polyubiquitin chain of a substrate protein binds to the UBA domain of p62, the substrate can be trafficked to the proteasome for degradation. An example of this is the p62-dependent turnover of tau which requires p62 for proteasomal degradation [21]. p62 may also be involved the autophagic/lysosomal pathway by sequestering aggregated proteins prior to their inclusion in autophagosomes.
