Abstract
Colorectal cancers with high-frequency microsatellite instability show peculiar clinicopathological features and a favorable clinical outcome. We investigated whether the improved prognosis for these cancers is related to the content of activated cytotoxic intraepithelial T lymphocytes. Microsatellite instability and the amount of activated cytotoxic lymphocytes were analyzed according to clinicopathological features, survival, and disease recurrence in 109 right-sided colon carcinomas from 245 consecutive patients with stage II/III colon cancer that underwent radical surgery. High-frequency microsatellite instability was found in 43% of stage II/III proximal colon cancers and was associated with significantly higher numbers of activated cytotoxic lymphocytes. High-frequency microsatellite instability, as well as the content of intratumoral-activated cytotoxic T lymphocytes correlated with improved overall and disease-free survival, particularly in patients with stage III tumors. Multivariate analysis revealed that patients with both features had a risk of death and relapse markedly lower than that associated with microsatellite status or intratumoral cytotoxic lymphocytes separately. The presence of local cytotoxic immune responses is probably the major determinant of the good clinical course of patients with microsatellite unstable colon cancer. Furthermore, high-frequency microsatellite instability coupled with a high content of intratumoral cytotoxic lymphocytes may identify a subset of colon cancer patients with a favorable clinical outcome, particularly in stage III disease.
Despite earlier diagnosis and improvements in therapeutic procedures, colorectal cancer is far from being completely controlled by conventional approaches, with a 5-year relative survival rate of 48%. 1,2 In these patients, assessment of prognosis mainly relies on the accurate staging of the disease. In particular, patients with TNM stage I colorectal cancer are potentially cured by surgery alone, whereas stage IV disease is only amenable to palliative care in most cases. 3 However, the clinical outcome of patients with stage II and III disease, accounting for more than 50 to 60% of all colorectal cancers, is currently not predictable for individual patients. The introduction of adjuvant chemotherapy further enhanced the need for prognostic factors able to discriminate between patients at low risk and high risk of relapse. In particular, the efficacy of additional therapy in patients with stage II disease is still under debate. 4 Furthermore, although adjuvant 5-fluorouracil-based regimens are the choice treatment for patients with stage III colorectal cancer, a proportion of these cases fails despite therapy. On these grounds, a better characterization of the biological features of these tumors may lead to the identification of additional and powerful prognostic factors for patients with stage II and III large bowel cancer.
Different genetic pathways have been identified along which colorectal cancer develops and progresses by a stepwise accumulation of widespread molecular alterations. 5,6 Recent evidence indicates that a subset of colorectal tumors are characterized by microsatellite instability (MSI), a genetic defect leading to the progressive accumulation of insertion/deletion mutations at simple repeated sequences (microsatellite sequences) as a consequence of impaired postreplicative DNA mismatch repair. 7,8 Patients with hereditary nonpolyposis colorectal cancer carry germline mutations of genes involved in DNA mismatch repair and these alterations are responsible for this mutator phenotype. 9 Furthermore, MSI is also found in ∼10 to 20% of sporadic colorectal cancers showing inactivation of DNA mismatch repair genes by somatic mutations or epigenetic silencing. 10,11 Colorectal tumors with high-frequency MSI (MSI-H), both hereditary nonpolyposis colorectal cancer-related and sporadic, are characterized by preferential location in the proximal colon, diploid nuclear DNA content, high prevalence of mucinous and medullary histotypes, and poor differentiation. 12,13 Moreover, recent studies reported that patients with MSI colonic cancers have a better clinical course. 14,15 One possible explanation for the favorable outcome of these patients is that the enhanced mutation rate associated with MSI, besides favoring mutations at genes critical for oncogenesis, may also induce a mutation burden no longer compatible with tumor cell survival. On the other hand, the peculiar genetic instability of MSI-H tumors may also lead to a continuous production of abnormal peptides that, by acting as neoantigens, could elicit specific antitumor immune responses potentially effective in limiting tumor growth and/or spread. In support of this hypothesis is the pronounced inflammatory infiltrate with a high content of tumor infiltrating lymphocytes, observed in MSI-H colorectal carcinomas. 16 Moreover, the presence of an antitumor cytotoxic immune response may have a favorable impact on the clinical outcome of patients with colorectal carcinomas. In fact, irrespective of the presence of MSI, a high content of cytotoxic effectors infiltrating these tumors strongly correlated with better survival rates. 17,18
Activation of CD8+ cytotoxic lymphocytes (CTLs) is characterized by the expression of peculiar proteins, such as perforin and granzyme B, involved in the induction of apoptosis in target cells. We have recently reported that MSI-H colorectal carcinomas carried significantly higher numbers of activated cytotoxic intraepithelial lymphocytes (IELs) than low frequency MSI (MSI-L) or microsatellite stable (MSS) tumors. 19 Moreover, evidence consistent with the presence of local antitumor cytotoxic immune responses in most MSI-H cases was reported. 19 On these grounds, it seems of relevance to assess whether the number of activated cytotoxic IELs is related to improved prognosis in patients with MSI-H colonic tumors. The present study was designed to address this issue and, in particular, to verify whether the combined evaluation of MSI and number of activated cytotoxic IELs may allow a more precise prediction of the clinical outcome of patients with stage II and III sporadic colon carcinomas.
Materials and Methods
Patients
Between January 1988 and December 1992, 245 consecutive patients with stage II and III colon carcinomas underwent radical surgery at a single institution (Arcispedale S. Anna, Ferrara, Italy). Among these, 118 showed disease located in the proximal colon, ie, from caecum to splenic flexure, were included in the study. In nine cases tumor tissue was not available. Selection for proximal colon cancer cases was justified by the need to enrich in MSI-H cases, because ∼90% of these tumors are located in the right portion of the colon. 14 Adjuvant monochemotherapy with 5-fluorouracil was administered to 22 patients (7 patients with stage II disease and 15 with stage III disease). Family history of colorectal cancer was excluded in all cases except one, showing early onset disease and a first-degree relative with colorectal neoplasia, although the Amsterdam criteria for hereditary nonpolyposis colorectal cancer were not fulfilled. 20 DNA ploidy analysis was performed in 47 tumor samples for which frozen tissue was available, as previously described. 21 All patients were followed-up until death or until December 1998. Information about the clinical outcome was obtained by hospital chart review or direct telephone interview with the patients’ personal physicians. Periodic controls included clinical examination, blood test (including serum carcinoembryonic antigen), endoscopy, abdominal ultrasonography, and radiography of the thorax. Computed tomography and magnetic resonance imaging were also performed when tumor recurrence was suspected.
Analysis of MSI
DNA was extracted from formalin-fixed, paraffin-embedded or frozen tissue, when available. Tumor specimens were chosen by microscopic examination, carefully avoiding areas enriched in nonneoplastic cells and necrotic debris. Tumor and corresponding normal DNA samples were analyzed for MSI at 5 to 10 microsatellite loci. Polymerase chain reaction was performed as previously described. 22 In agreement with Boland and colleagues, 23 all samples were evaluated for MSI at BAT25, BAT26, D17S250, D2S123, and D5S346, as a first reference panel. A further five microsatellites, including BAT40, D10S197, D18S58, D18S69, and L-myc, were analyzed when only one locus from the first panel showed MSI or when one or more loci were not evaluable. Tumors were scored as having MSI-H when additional bands were present in the polymerase chain reaction products from at least two loci of the first panel or from at least 30% of all examined loci, if additional microsatellites were analyzed. Tumors were scored as having MSS/MSI-L when any (MSS) or <30% (MSI-L) of the examined loci showed additional bands.
Histopathological Analysis
Conventional histopathological parameters, including AJCC/UICC TNM stage, tumor type, and grade of differentiation, were independently assessed by two pathologists (Giovanni Lanza and Roberta Gafá) without any knowledge of the results of genetic analysis. Tumors were typed as adenocarcinomas or mucinous adenocarcinomas according to the criteria of the World Health Organization. 24 Poorly differentiated adenocarcinomas with a predominantly solid growth pattern (at least 70% of the tumor area) and lack of marked nuclear pleomorphism were classified as medullary adenocarcinomas. 25 Grade of differentiation (well/moderate or poor) was determined following the World Health Organization guidelines. 24
Immunohistochemical Analysis
Immunohistochemical analyses were performed as previously described. 19 The primary antibodies used were: anti-CD3 (polyclonal, 1:1000) and anti-CD8 (clone cd8/144b, 1:25), purchased from DAKO (Glostrup, Denmark); and anti-granzyme B (clone GrB7, 1:20) obtained from Monosan (Leiden, The Netherlands). The number of IELs positive at immunohistochemistry as well as the number of tumor cells, assessed by morphology, were recorded in the same microscopic fields using a video-assisted cell analysis system (MicroImage; SC Casti Imaging, Venezia, Italy). The ratio between the number of positive IELs and that of tumor cells associated with was then calculated. At least five randomly selected high-power microscopic fields were evaluated, accurately avoiding necrotic and superficial areas. In a limited number of cases, immunohistochemical evaluation was performed on sections from two different blocks.
Statistical Analysis
The index date for survival calculation was defined as the date of diagnostic confirmation for colon cancer. The months of observation were calculated from the index date to the date of last information/death. The observed survival was estimated using the Kaplan and Meier product limit method, and cumulative survival probability was calculated at 5 years stratified according to MSI phenotype and stage at diagnosis. Differences were tested using the log-rank test. To assess the relative excess risk of death/recurrence according to MSI status and type of activated cytotoxic IELs and to control for confounding factors (age as continuous variable, gender, and pathological stage), proportional hazards models were fitted by computing hazard ratios and the corresponding 95% confidence intervals (95% CI). Mean cell count was analyzed dividing the distributions of CD3+, CD8+, and granzyme B+ IELs into two groups of equal size, using the median value as cut-point. The proportional assumption was examined with log-log survival plots or by adding time-dependent interaction terms into the model. Interaction terms between MSI phenotype and type of activated cytotoxic IELs divided according to the median value were calculated by means of the Wald chi-square test with one degree of freedom.
Results
Clinicopathological Features of MSI-H Colonic Tumors
Forty-seven patients (43.1%) showed MSI-H tumors, and 62 patients (56.9%) had MSS/MSI-L tumors (48 MSS and 14 MSI-L). MSI-H tumors were more frequent in patients older than 60 years (87.2% versus 69.4%; P value, 0.03) than MSS/MSI-L tumors, whereas no difference in terms of sex and stage distribution was observed (Table 1) ▶ .
Table 1.
Distribution of Patients’ Clinicopathological Characteristics According to MSI Status
| Characteristic | All patients (n = 109) | MSS/MSI-L (n = 62) | MSI-H (n = 47) | P value* |
|---|---|---|---|---|
| Age (mean-SD) | 66.4–10.1 | 69.3–12.4 | ||
| Class of age, no. (%) | ||||
| <60 | 25 (22.9) | 19 (30.7) | 6 (12.8) | |
| ≥60 | 84 (77.1) | 43 (69.4) | 41 (87.2) | 0.03 |
| Sex, no. (%) | ||||
| Males | 45 (41.3) | 25 (40.3) | 20 (42.6) | |
| Females | 64 (58.7) | 37 (59.7) | 27 (57.5) | 0.8 |
| Pathological stage, no. (%) | ||||
| II | 55 (50.5) | 28 (45.2) | 27 (57.5) | |
| III | 54 (49.5) | 34 (54.8) | 20 (42.6) | 0.2 |
| Grade, no. (%) | ||||
| Well/Moderate | 64 (58.7) | 45 (72.6) | 19 (40.4) | |
| Poor | 45 (41.3) | 17 (27.4) | 28 (59.6) | 0.0009 |
| Histological type, no. (%) | ||||
| Adenocarcinoma NOS | 77 (70.6) | 52 (83.9) | 25 (53.2) | |
| Mucoid adenocarcinoma | 28 (25.7) | 10 (16.1) | 18 (38.3) | |
| Solid type | 4 (3.7) | − (0.0) | 4 (8.5) | 0.0009 |
| Ploidy, no. (%) | ||||
| Diploid | 22 (46.8) | 6 (24.0) | 16 (72.7) | |
| Aneuploid | 25 (53.2) | 19 (76.0) | 6 (27.3) | 0.001 |
| Adjuvant chemotherapy, no. (%) | ||||
| No | 87 (79.8) | 49 (79.0) | 38 (80.9) | |
| Yes | 22 (20.2) | 13† (21.0) | 9‡ (19.2) | 1.0 |
| Life status, no. (%) | ||||
| Alive | 72 (66.1) | 33 (53.2) | 39 (82.9) | |
| Dead | 37 (33.9) | 29 (46.8) | 8 (17.0) | 0.001 |
| Recurrence, no. (%) | ||||
| No | 74 (67.9) | 35 (56.5) | 39 (82.9) | |
| Yes | 36 (33.1) | 27 (43.6) | 8 (17.0) | 0.004 |
*Fisher’s exact test.
†Four patients had stage II disease, 9 had stage III disease.
‡Three patients had stage II disease, 6 had stage III disease.
Histopathological analysis showed that MSI-H colonic cancer more frequently had a mucoid differentiation than MSS/MSI-L cancers (38.3% versus 16.1%); the four cases showing a solid/medullary histotype were MSI-H. Furthermore, MSI-H tumors were significantly more often poorly differentiated compared with MSS/MSI-L tumors (59.6% versus 27.4%; P value, 0.0009). DNA ploidy analysis was available in 47 cases and MSI-H tumors prevalently showed a diploid nuclear DNA content (72.7% versus 24.0%; P value, 0.001).
The median follow-up time among patients alive as of their last follow-up was 74 months (range, 50 to 120 months) and 78 months (range, 46 to 108 months) for patients with MSS/MSI-L and MSI-H tumors, respectively. Adjuvant chemotherapy was administered only to a fraction of patients (22 of 109, 20.2%), the majority of which had stage III disease (15 of 22, 68.1%); cases with MSI-H were not differently treated compared to cases with MSS/MSI-L tumors (19.2% versus 21.0%).
Any difference in the distribution of clinical and pathological features was found between MSS and MSI-L cases.
Phenotypic Characterization of Tumor-Associated IELs
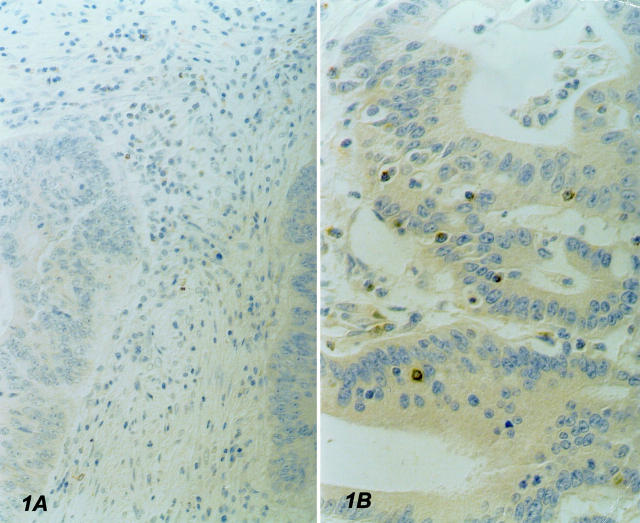
In all cases, IEL density was fairly uniform throughout the nonnecrotic areas of tumor samples. No correlation was found between the number of IELs and sex, age, and TNM stage, except for a borderline association between stage and CD8+ IEL number, with a slightly lower prevalence among TNM stage III cases (data not shown). MSI-H tumors carried a higher number of CD3+ intraepithelial T lymphocytes than MSS/MSI-L tumors (mean SD: 0.057-0.043 versus 0.043-0.025; P value, 0.05). Accordingly, a significantly higher prevalence of CD8+ IELs was observed in MSI-H than in MSS/MSI-L tumors (mean SD: 0.049-0.045 versus 0.033-0.023; P value, 0.02), indicating a more efficient recruitment of cytotoxic precursors. Of note, a large proportion of these cytotoxic precursors was activated, as shown by the higher content of GrB+ IELs observed in MSI-H cases (mean-SD: 0.020-0.01 versus 0.012-0.01; P value, 0.0001) (Figure 1) ▶ .
Figure 1.
Immunostaining with anti-granzyme B antibody. A: MSS colon cancer showing no evidence of GrB+ IELS; rare positive cells are present in the stroma. B: MSI-H tumor carrying high numbers of GrB+ IELs. Original magnifications, ×250.
Survival Analysis According to the MSI Status
Of the 109 patients investigated, 72 (66%) were still alive at the end of the study, with a mean follow-up of 78 months (range, 46 to 120 months). During the observation period, 37 deaths were recorded, 17 of which occurred in the first 2 years from diagnosis. The overall survival probability was 0.85 at 2 years and 0.65 at 5 years of observation. Patients with MSI-H tumors had a better clinical course, with significantly higher 5-year overall survival (82% versus 53%; P value, 0.001) and disease-free rates (82% versus 55%; P value, 0.002) than patients with MSS/MSI-L.
Stratification according to TNM staging further confirmed these findings. In fact, MSI-H patients with stage III disease had significantly better overall survival (72% versus 35%; P value, 0.006) and relapse-free rates (66% versus 39%; P value, 0.02) than the corresponding MSS/MSI-L patients (Table 2) ▶ . The occurrence of MSI-H was also correlated with improved clinical outcome in patients with stage II disease, although differences were not statistically significant, as shown by 5-year overall survival (89% versus 75%; P value, 0.2) and relapse-free rates (93% versus 75%; P value, 0.08) (Table 2) ▶ .
Table 2.
Five-Year Cumulative Probability for Survival and Relapse, and Hazard Ratios from Cox Proportional Hazard Regression Models According to MSI Status
| 5-year cumulative probability (95% CI) | Log-rank test chi-square (P value) | HR* (95% CI) | ||
|---|---|---|---|---|
| MSS/MSI-L (n = 62) | MSI-H (n = 47) | |||
| Survival | ||||
| Stage | ||||
| II | 0.75 (0.59–0.91) | 0.89 (0.77–1.00) | 1.91 (0.2) | 0.33 (0.08–1.31) |
| III | 0.35 (0.19–0.51) | 0.72 (0.51–0.93) | 7.4 (0.006) | 0.29 (0.11–0.76) |
| Overall | 0.53 (0.40–0.65) | 0.82 (0.71–0.93) | 10.77 (0.001) | 0.31 (0.14–0.68) |
| Relapse | ||||
| Stage | ||||
| II | 0.75 (0.57–0.91) | 0.93 (0.83–1.00) | 3.16 (0.08) | 0.23 (0.05–1.15) |
| III | 0.39 (0.22–0.56) | 0.66 (0.44–0.89) | 5.26 (0.02) | 0.35 (0.14–0.89) |
| Overall | 0.55 (0.43–0.68) | 0.82 (0.71–0.93) | 9.59 (0.002) | 0.32 (0.15–0.72) |
*Estimates from separate proportional hazard regression models including terms for sex, age, and stage as appropriate. Reference category was represented by cases MSS/MSI-L.
Multivariate analysis adjusting for sex, age as continuous, and TNM stage confirmed that MSI-H was significantly and independently associated with a better prognosis. In fact, patients with MSI-H tumors showed hazard ratios of 0.31 (95% CI, 0.14 to 0.68) for death and 0.32 (95% CI, 0.15 to 0.72) for relapse (Table 2) ▶ .
Prognostic Value of the Content of Activated Cytotoxic IELs
The prognostic significance of the number of activated cytotoxic IELs was first investigated by univariate survival analysis, classifying each case as having high or low CD3+, CD8+, and GrB+ cell count according to the median value. Results showed that high levels of CD3+, CD8+, and GrB+ IELs were associated with improved survival (Table 3) ▶ . Multivariate analysis also confirmed that the content of CD3+, CD8+, and GrB+ IELs positively affected the clinical outcome, independently of age, sex, and TNM stage (Table 3) ▶ .
Table 3.
Five-Year Cumulative Probability for Survival and Relapse According to the Number of CD3+, CD8+, and GrB+ IELs Stratified Using Median Value as Cut-Point
| 5 Year cumulative probability (95% CI) | Log-rank test chi-square (P value) | HR* (95% CI) | ||
|---|---|---|---|---|
| Below median | Above median | |||
| CD3+ IELs | ||||
| Survival | ||||
| Stage | ||||
| II | 0.77 (0.60–0.95) | 0.87 (0.76–0.99) | 1.04 (0.3) | 0.50 (0.13–1.94) |
| III | 0.39 (0.21–0.57) | 0.70 (0.49–0.90) | 4.79 (0.03) | 0.37 (0.14–0.93) |
| Overall | 0.55 (0.42–0.69) | 0.80 (0.70–0.91) | 8.22 (0.004) | 0.40 (0.19–0.85) |
| Relapse | ||||
| Stage | ||||
| II | 0.77 (0.60–0.95) | 0.91 (0.80–1.00) | 1.73 (0.2) | 0.40 (0.09–1.76) |
| III | 0.42 (0.22–0.61) | 0.65 (0.44–0.86) | 2.96 (0.09) | 0.45 (0.18–1.11) |
| Overall | 0.58 (0.44–0.72) | 0.80 (0.70–0.91) | 6.48 (0.01) | 0.42 (0.19–0.92) |
| CD8+ IELs | ||||
| Survival | ||||
| Stage | ||||
| II | 0.64 (0.44–0.84) | 0.94 (0.91–1.00) | 8.07 (0.005) | 0.14 (0.03–0.65) |
| III | 0.44 (0.26–0.62) | 0.63 (0.42–0.85) | 2.10 (0.1) | 0.50 (0.20–1.22) |
| Overall | 0.52 (0.39–0.66) | 0.82 (0.72–0.93) | 11.16 (0.0008) | 0.33 (0.15–0.73) |
| Relapse | ||||
| Stage | ||||
| II | 0.68 (0.48–0.88) | 0.94 (0.86–1.00) | 6.52 (0.01) | 0.17 (0.04–0.83) |
| III | 0.42 (0.23–0.62) | 0.64 (0.43–0.86) | 1.71 (0.2) | 0.47 (0.19–1.17) |
| Overall | 0.54 (0.40–0.68) | 0.83 (0.73–0.93) | 9.55 (0.002) | 0.35 (0.16–0.78) |
| GrB+ IELs | ||||
| Survival | ||||
| Stage | ||||
| II | 0.64 (0.45–0.83) | 0.96 (0.90–1.00) | 9.48 (0.002) | 0.07 (0.01–0.53) |
| III | 0.29 (0.12–0.46) | 0.72 (0.54–0.90) | 7.52 (0.006) | 0.32 (0.14–0.77) |
| Overall | 0.46 (0.32–0.59) | 0.85 (0.76–0.95) | 17.48 (0.0001) | 0.23 (0.10–0.50) |
| Relapse | ||||
| Stage | ||||
| II | 0.64 (0.44–0.83) | 1.00 (1.00–1.00) | 12.73 (0.0004) | † |
| III | 0.28 (0.10–0.46) | 0.72 (0.54–0.89) | 7.12 (0.008) | 0.33 (0.14–0.79) |
| Overall | 0.46 (0.32–0.60) | 0.87 (0.78–0.96) | 18.71 (0.0001) | 0.20 (0.09–0.46) |
*Estimates from separate proportional hazard regression models including terms for sex, age, and stage as appropriate. Reference category was represented by cases below the median value.
†No events.
Furthermore, analysis of the number of activated cytotoxic IELs according to MSI status showed that high numbers of CD3+, CD8+, and GrB+ IELs, stratified according to the median value, were significantly associated with a lower risk of death in both MSS/MSI-L and MSI-H cases (Table 4) ▶ . All interaction terms estimated between MSI phenotype and CD3+, CD8+, and GrB+ cells subdivided according to the median were highly significant.
Table 4.
Hazard Ratios from Cox Proportional Hazard Regression Models According to MSI Status and Number of CD3+, CD8+, and GrB+ IELs Stratified Using Median Value as Cut-Point
| MSS/MSI-L (n = 62) | MSI-H (n = 47) | Overall HR (95% CI) | |
|---|---|---|---|
| CD3+ IELs (mean-SD) | |||
| Below median (0.03–0.008) | 1# | 0.22 (0.08–0.65) | 1# |
| Above median (0.07–0.04) | 0.32 (0.13–0.79) | 0.13 (0.04–0.45) | 0.40 (0.19–0.85) |
| CD8+ IELs (mean-SD) | |||
| Below median (0.02–0.007) | 1# | 0.34 (0.12–0.90) | 1# |
| Above median (0.06–0.04) | 0.38 (0.16–0.94) | 0.09 (0.02–0.39) | 0.33 (0.15–0.73) |
| GrB+ IELs (mean-SD) | |||
| Below median (0.007–0.004) | 1# | 0.82 (0.33–2.05) | 1# |
| Above median (0.02–0.007) | 0.48 (0.19–1.18) | 0.09 (0.02–0.35) | 0.23 (0.10–0.50) |
| Overall HR (95% CI) | 1# | 0.31 (0.14–0.68) |
#, Reference category.
In each cell we reported HR (95% CI) from separate models including terms for age, sex, and stage.
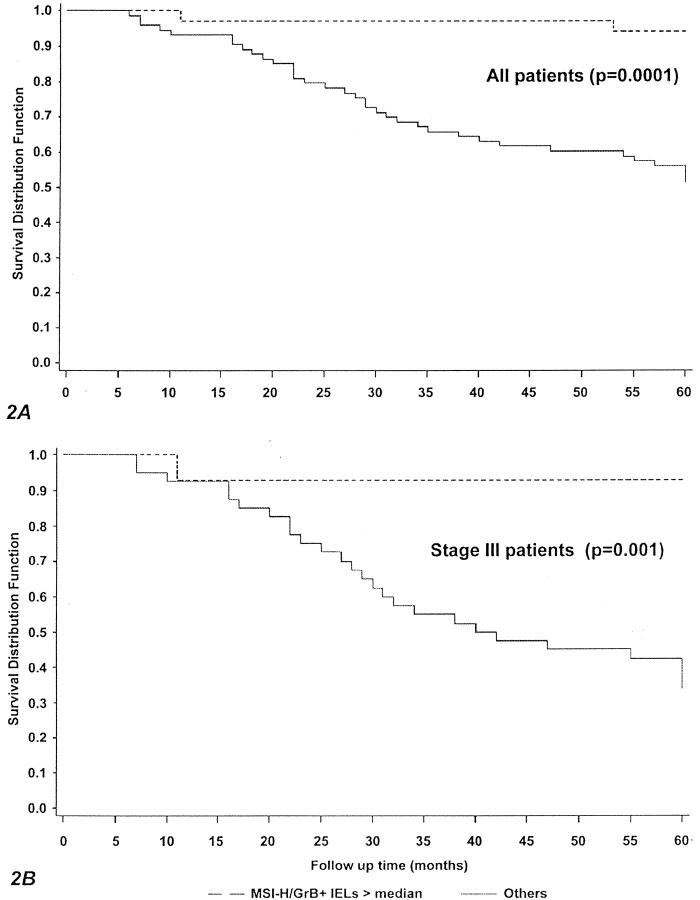
Furthermore, and more interestingly, the risk of death associated with the presence of both MSI-H and a high content of CD3+, CD8+, and GrB+ IELs was always markedly lower than that contributed by MSI-H or high cell counts separately, identifying a subset of cases with a risk of death significantly decreased (of ∼90%) compared with the reference category (MSS/MSI-L cases with low CD3+, CD8+, and GrB+ cell count). Consistently, patients carrying tumors with both MSI-H and high GrB+ cell counts had significantly more favorable 5-year overall survival (94% versus 51%; P value, 0.0001) (Figure 2A) ▶ and relapse-free rates (94% versus 53%; P value 0.0001) (not shown) than all of the other patients. Of note, when only stage III cases were investigated, differences between the two subgroups were even more pronounced, with 5-year overall survival (93% versus 34%; P value, 0.001) (Figure 2B) ▶ and relapse-free rates (86% versus 36%; P value, 0.001) (not shown) significantly better in patients with both MSI-H and high content of GrB+ IELs.
Figure 2.
Kaplan-Meier survival curves according to MSI status and GrB+ IELs. A: All cases. B: TNM stage III cases.
Discussion
The results herein reported confirm and extend previous findings indicating that MSI-H colorectal carcinomas constitute a distinct subset of tumors characterized by specific biological and clinicopathological features. 12-16 In particular, we confirm, in an unrelated series, our previous observation that most MSI-H colonic carcinomas carry high numbers of activated cytotoxic IELs, consistent with the presence of cytotoxic antitumor immune responses. Our results also indicate that these local cell-mediated immune responses may have clinical relevance. In fact, despite features otherwise indicating a poorer outcome (eg, poor differentiation), patients with MSI-H tumors had a more favorable prognosis than MSI-L/MSS cases, particularly those with stage III disease.
The most relevant finding comes from the combined evaluation of MSI and the content of activated cytotoxic IELs. Such an approach allowed the identification of patients with both MSI-H tumors and high numbers of activated cytotoxic effectors as a distinct subset of cases characterized by a very good clinical course. These patients, comprising ∼30% of our series of right-sided colonic carcinomas, had a risk of death of ∼10% of that observed in patients with MSS tumors carrying low numbers of activated cytotoxic IELs, independent of TNM stage, sex, and age. Of note, a quasi-multiplicative interaction in favorably influencing the clinical outcome was found between MSI-H and high numbers of activated CTLs infiltrating cancer cell nests. Consistently, this interaction resulted in markedly improved overall survival and relapse-free times in the whole series, and these differences were even more pronounced in patients with stage III disease.
The finding that only MSI-H cases with a high content of activated cytotoxic IELs have a better prognosis further supports the hypothesis that the presence of local antitumor immune response is probably one of the major determinants of the more favorable course observed in patients with MSI-H tumors. Therefore, it is likely that in most MSI-H cases, the continuous production of abnormal peptides favored by the inherent genetic instability of tumor cells is responsible for the induction of clinically relevant cytotoxic immune responses.
Our findings, suggesting the less aggressive clinical behavior of MSI-H colonic tumors is likely because of a better local immune control of the disease, may have relevant implications for the management of these patients. In fact, our observation that cases showing both MSI-H and a high content of activated cytotoxic IELs have a very favorable prognosis questions the need for adjuvant chemotherapy in these patients. This is a very controversial issue. In fact, recent articles reported opposite effects of chemotherapy in MSI-H colorectal cancers. In particular, a recent large retrospective study indicated that patients with MSI-H Duke’s stage C colorectal cancer did not have a favorable outcome unless they had received adjuvant chemotherapy. 26 Furthermore, Hemminki and colleagues 27 reported a better prognosis in patients with MSI-H cancer who had received adjuvant therapy. Nevertheless, in the first work patients were stratified according to the Duke’s staging system and therefore it cannot be ruled out that some MSI-H cases with distant metastases may have not received any additional therapy after surgery. It is worth considering that, in the present series, additional therapy was administered only to a minority of cases (∼20%), with an even distribution of stage II and III disease in patients with either MSI-H or MSS/MSI-L tumors. In keeping with our data, Wright and colleagues 28 showed that, in patients with locally advanced colorectal cancer who have not received additional chemotherapy, MSI-H is an independent positive prognostic factor. In addition, recent in vitro evidence indicates that MSI-H colon cancer cell lines are resistant to 5-fluorouracil at concentrations similar to those achieved in patients’ tissues. 29 On these grounds, our results provide the rationale for further studies aimed at defining the real usefulness of adjuvant chemotherapy in the treatment of most patients with MSI-H colon cancer, particularly in stage III disease.
Acknowledgments
We thank Ms. Fernanda Mora, Ms. Daniela Nardo, and Mr. Eros Magri for their helpful work.
Footnotes
Address reprint requests to Dr. Mauro Boiocchi, Division of Experimental Oncology 1, Centro di Riferimento Oncologico, via Pedemontana Occidentale, 12-33081 Aviano (PN), Italy. E-mail: mboiocchi@cro.it.
Supported in part by a grant from Associazione Italiana per la Ricerca sul Cancro.
References
- 1.Parkin DM, Pisani P, Ferlay J: Estimates of the worldwide incidence of twenty-five major cancers in 1990. Int J Cancer 1999, 80:827-841 [DOI] [PubMed] [Google Scholar]
- 2.Berrino F, Sant M, Verdecchia A: Survival of cancer patients in Europe. 1995. IARC, IARC Scientific Publication 132, Lyon [PubMed]
- 3.American Joint Committee on Cancer: Manual for Staging of Cancer, 4th ed. Edited by OH Beahrs, DE Henson, RV Hutter, BJ Kennedy. Philadelphia, JB Lippincott Company, 1992
- 4.Moore HCF, Haller DG: Adjuvant therapy of colon cancer. Semin Oncol 1999, 26:545-555 [PubMed] [Google Scholar]
- 5.Fearon ER, Vogelstein B: A genetic model for colorectal tumorigenesis. Cell 1990, 61:759-767 [DOI] [PubMed] [Google Scholar]
- 6.Ilyas M, Straub J, Tomlinson IPM, Bodmer WF: Genetic pathways in colorectal and other cancers. Eur J Cancer 1999, 35:335-351 [DOI] [PubMed] [Google Scholar]
- 7.Thibodeau SN, Bren G, Schaid D: Microsatellite instability in cancer of the proximal colon. Science 1993, 260:812-816 [DOI] [PubMed] [Google Scholar]
- 8.Ionov Y, Peinado MA, Malkhosyan S, Shibata D, Perucho M: Ubiquitous somatic mutations in simple repeated sequences reveal a new mechanism for colon carcinogenesis. Nature 1993, 363:816-819 [DOI] [PubMed] [Google Scholar]
- 9.Lynch HT, Smyrk T: An update on Lynch syndrome. Curr Opin Oncol 1998, 10:349-356 [DOI] [PubMed] [Google Scholar]
- 10.Wu Y, Nystrom-Lahti M, Osinga J, Looman MWG, Peltomaki P, Aaltonen LA, de la Chapelle A, Hofstra RMW, Buys CHCM: MSH2 and MLH1 mutations in sporadic replication error-positive colorectal carcinoma as assessed by two-dimensional DNA electrophoresis. Genes Chromosom Cancer 1997, 18:269-278 [PubMed] [Google Scholar]
- 11.Kane MF, Loda M, Gaida GM, Lipman J, Mishra R, Goldman H, Jessup JM, Kolodner R: Methylation of the hMLH1 promoter correlates with lack of expression of hMLH1 in sporadic colon tumors and mismatch repair-defective human tumor cell lines. Cancer Res 1997, 57:808-811 [PubMed] [Google Scholar]
- 12.Risio M, Reato G, Francia di Celle P, Fizzotti M, Rossini FP, Foà R: Microsatellite instability is associated with the histological features of the tumor in nonfamilial colorectal cancer. Cancer Res 1996, 56:5470-5474 [PubMed] [Google Scholar]
- 13.Jass JR, Do K-A, Simms LA, Iino H, Wynter C, Pillay SP, Searle J, Radford-Smith G, Young J, Leggett B: Morphology of sporadic colorectal cancer with DNA replication errors. Gut 1998, 42:673-679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gryfe R, Kim H, Hsieh ETK, Aronson MD, Holowaty EJ, Bull SB, Redston M, Gallinger S: Tumor microsatellite instability and clinical outcome in young patients with colorectal cancer. N Engl J Med 2000, 342:69-77 [DOI] [PubMed] [Google Scholar]
- 15.Halling KC, French AJ, McDonnell SK, Burgart LJ, Schaid DJ, Peterson BJ, Moon-Tasson L, Mahoney MR, Sargent DJ, O’Connell MJ, Witzig TE, Farr GH, Jr, Goldberg RM, Thibodeau SN: Microsatellite instability and 8p allelic imbalance in stage B2 and C colorectal cancers. J Natl Cancer Inst 1999, 91:1295-1303 [DOI] [PubMed] [Google Scholar]
- 16.Kim H, Jen J, Vogelstein B, Hamilton SR: Clinical and pathological characteristic of sporadic colorectal carcinomas with DNA replication errors in microsatellite sequences. Am J Pathol 1994, 145:148-156 [PMC free article] [PubMed] [Google Scholar]
- 17.Naito Y, Saito K, Shiiba K, Ohuchi A, Saigenji K, Nagura H, Ohtani H: CD8+ T cells infiltrated within cancer cell nests as a prognostic factor in human colorectal cancer. Cancer Res 1998, 58:3491-3494 [PubMed] [Google Scholar]
- 18.Ropponen KM, Eskelinen MJ, Lipponen PK, Alhava E, Kosma V-M: Prognostic value of tumour-infiltrating lymphocytes (TILs) in colorectal cancer. J Pathol 1997, 182:318-324 [DOI] [PubMed] [Google Scholar]
- 19.Dolcetti R, Viel A, Doglioni C, Russo A, Guidoboni M, Capozzi E, Vecchiato N, Macrì E, Fornasarig M, Boiocchi M: High prevalence of activated intraepithelial cytotoxic T Lymphocytes and increased neoplastic cell apoptosis in colorectal carcinomas with microsatellite instability. Am J Pathol 1999, 154:1805-1813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vasen HFA, Mecklin J-P, Meera Khan P, Lynch HT: The International collaborative group on hereditary non-polyposis colorectal cancer. Dis Colon Rectum 1991, 34:424-425 [DOI] [PubMed] [Google Scholar]
- 21.Lanza G, Gafà R, Santini A, Maestri I, Dubini A, Gilli G, Cavazzini L: Prognostic significance of DNA ploidy in patients with stage II and III colon carcinoma. Cancer 1998, 82:49-59 [DOI] [PubMed] [Google Scholar]
- 22.Viel A, Dell’Agnese L, Canzonieri V, Sopracordevole F, Capozzi E, Carbone A, Visentin MC, Boiocchi M: Suppressive role of the metastasis-related nm23–H1 gene in human ovarian carcinoma: association of high messengers RNA expression with lack of lymph node metastasis. Cancer Res 1995, 55:2645-2650 [PubMed] [Google Scholar]
- 23.Boland CR, Thibodeau SN, Hamilton SR, Sidranski D, Eshleman JR, Burt RW, Meltzer SJ, Rodriguez-Bigas MA, Fodde R, Ranzani GN, Srivastava S: A National Cancer Institute workshop on microsatellite instability for cancer detection and familial predisposition: development of international criteria for the determination of microsatellite instability in colorectal cancer. Cancer Res 1998, 58:5248-5257 [PubMed] [Google Scholar]
- 24.Jass JR, Sobin LH: Histological Typing of Intestinal Tumors: World Health Organization, ed 2. New York, Springer-Verlag, 1989
- 25.Lanza G, Gafà R, Matteuzzi M, Santini A: Medullary-type poorly differentiated adenocarcinoma of the large bowel: a distinct clinicopathologic entity characterized by microsatellite instability and improved survival. J Clin Oncol 1999, 17:2429-2438 [DOI] [PubMed] [Google Scholar]
- 26.Elsaleh H, Joseph D, Grieu F, Zeps N, Spry N, Iacopetta B: Association of tumour site and sex with survival benefit from adjuvant chemotherapy in colorectal cancer. Lancet 2000, 355:1745-1750 [DOI] [PubMed] [Google Scholar]
- 27.Hemminki A, Mecklin J-P, Jarvinen H, Aaltonen LA: Microsatellite instability is a favorable prognostic indicator in patients with colorectal cancer receiving chemotherapy. Gastroenterology 2000, 119:921-928 [DOI] [PubMed] [Google Scholar]
- 28.Wright CM, Dent OF, Barker M, Newlands RC, Chapuis PH, Bokey EL, Young JP, Leggett BA, Jass JR, Macdonald GA: Prognostic significance of extensive microsatellite instability in sporadic clinicopathological stage C colorectal cancer. Br J Surg 2000, 87:1197-1202 [DOI] [PubMed] [Google Scholar]
- 29.Carethers JM, Chauhan DP, Fink D, Nebel S, Bresalier RS, Howell SB, Boland CR: Mismatch repair proficiency and in vitro response to 5-fluorouracil. Gastroenterology 1999, 117:123-131 [DOI] [PMC free article] [PubMed] [Google Scholar]