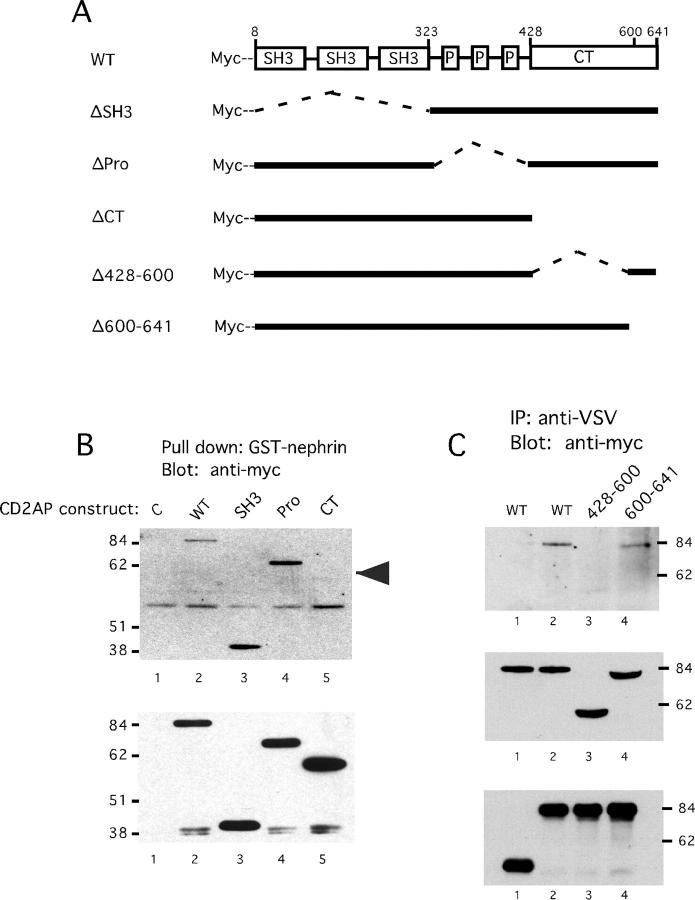
Figure 4.
Mapping the binding site for nephrin in CD2AP. A: Schematic diagram of CD2AP deletion mutants. ΔSH3 lacks residues 8–323 which contain the three SH3 domains. ΔPro lacks residues 324–427 containing the proline-rich domain. ΔCT lacks residues 428–636 encoding the C-terminal portion. Δ428–600 lacks residues 428–600 and Δ601–641 lacks residues 601–641. B: The C-terminal domain of CD2AP binds to nephrin. Wild-type or indicated CD2AP constructs were expressed in HeLa cells using the vaccinia-T7 expression. Cell lysates were incubated with GST-nephrin immobilized on agarose beads. Bound proteins were analyzed by immunoblotting with anti-myc. Uninfected HeLa cell lysate was used as control (lane 1). Whole cell lysates were also probed with anti-Myc to confirm equivalent CD2AP expression levels (lower panel). C: Residues 428–600 mediate binding of CD2AP to nephrin. Wild-type CD2AP, Æ428–600, Æ600–641 were co-expressed in HeLa cells with the VSV G/nephrin fusion protein (lanes 2–4). As a negative control, wild-type CD2AP was co-expressed with a truncated form of VSV G lacking the cytoplasmic domain of nephrin (lane 1). Immunoprecipitates were prepared with anti-VSV G antibody and analyzed by immunoblotting using anti-Myc to detect CD2AP. The same blot was reprobed using anti-VSV antisera to confirm expression levels of the VSV G/nephrin fusion protein (bottom). Whole cell lysates were also probed for CD2AP expression using Myc antibody (middle).