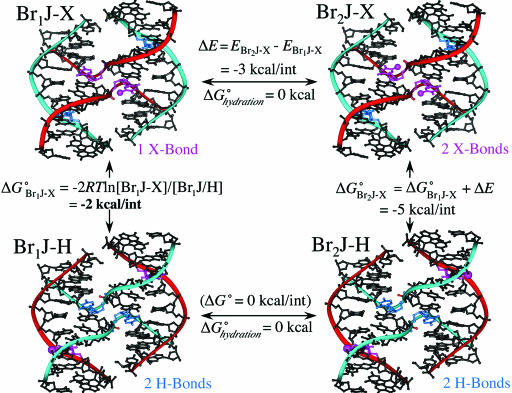
Fig. 4.
Thermodynamic cycle to estimate the free energies of the X- relative to H-bonds. A free energy difference of ≈1 kcal/mol was estimated between the X- and H-isomers from the occupancies of bromine at the inside and outside strands of Br1J (Br1J-X and Br1J-H) (Si Fig. 6) or ≈2 kcal/mol for 1 X-bond vs. 1 H-bond. Because there is very little contribution of hydration free energy to placing the bromine in the H- or X-isomeric forms (ΔGhydrationo ≈0), we can assume that the primary effect on the energy of the X-bonds is electrostatic. Finally, if we assume that the is no difference in the energies of the H-isomer for either the Br1J or Br2J constructs (Br1J-H and Br2J-H, bottom of cycle, with the bromines on the outside strands), then the energy of the X-bond in the Br2J construct (ΔG°Br2J-X) can be estimated as the sum the Br1J X-bond and the difference in electrostatic energy (ΔE) estimated from ab initio calculations (SI Fig. 7).