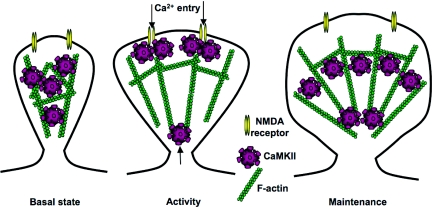
Fig. 6.
A model for the “gating” mechanism of synaptic structure by CaMKII. (Left) At resting synapses, actin is bundled by CaMKII, thereby maintaining a stable structure. (Center) When CaMKII is activated by neuronal activity and the resultant Ca2+ influx, it detaches from F-actin and allows its reorganization by other signal transduction machineries, such as small G proteins. Meanwhile, more CaMKII are recruited to the synapse by self-association or interaction with NR2B and plays a role as a signal transduction molecule. Such mechanism may set a new level for the amount of CaMKII at dendritic spines. (Right) Upon returning to the unphosphorylated state, CaMKII bundles the newly reorganized F-actin and maintains the remodeled spine structure. The new level of CaMKII may affect the amount of bundled F-actin in the spines, and consequently the structure of the dendritic spines.