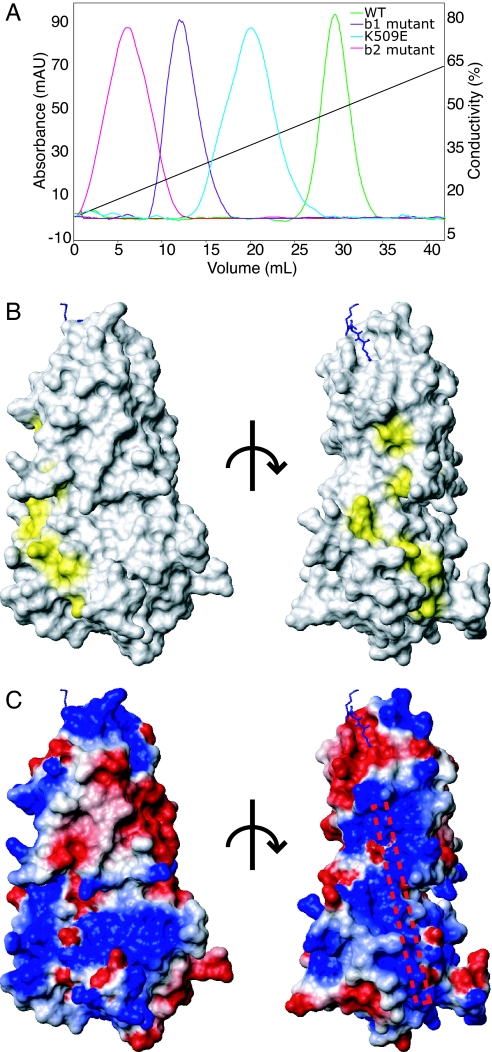
Fig. 4.
The tertiary structure of N1b1b2 domains creates a single heparin binding surface. (A) Heparin Sepharose binding of b1b2 mutants, which maps the surface involved in heparin binding. (B) Surface mapping of the residues identified as important for heparin binding using the orientation used in Fig. 1D (Left) and a 90° rotation about a vertical axis (Right). (C) Electrostatic surface map of N1b1b2 in the same orientations shown in B with a schematic rod marking the position of the heparin binding surface based on the observed electropositive surface patch and mutagenesis.