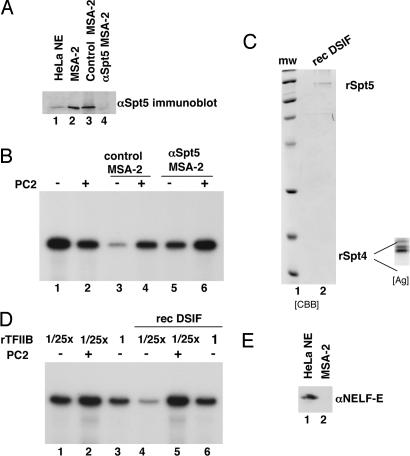
Fig. 4.
MSA-2 activity of DSIF. (A) The MSA-2 Ni-NTA agarose fraction was immunodepleted over an anti-Spt5-protein A Sepharose resin and analyzed by immunoblotting using anti-Spt5 antibodies (lane 4). A mock-depleted fraction (control MSA-2, lane 3) was also analyzed. (B) Transcriptional activity of anti-Spt5-depleted MSA-2 Ni-NTA agarose fraction. Mock (lanes 3 and 4) and anti-Spt5-depleted MSA-2 fractions (lanes 5 and 6) were analyzed for PC2 responsiveness in in vitro transcription reactions reconstituted as in Fig. 2D. HNF-4 was present in all reactions. RNA products were quantified by phosphorimaging (see Results). (C) DSIF was reconstituted from the individual recombinant proteins, which were corenatured. The resulting heterodimer was further purified by gel filtration and analyzed by SDS/PAGE and Coomassie staining (CBB). The bottom part of the gel was also stained with silver (Ag; Inset) to better visualize the smaller hSpt4 subunit, which was not easily seen with Coomassie. (D) Transcriptional assay of recombinant DSIF. In vitro transcription reactions were reconstituted as in legend to Fig. 2D except that variable amounts of TFIIB were used: 0.4 ng in lanes 1, 2, 4, and 5; 10 ng in lanes 3 and 6. PC2 and recombinant DSIF (rec DSIF) were added as indicated. (E) Immunoblot analysis to show absence of NELF (using antibodies against the NELF-E subunit) in the MSA-2 Ni-NTA agarose fraction. Lane 1, HeLa nuclear extract; lane 2, MSA-2 fraction.