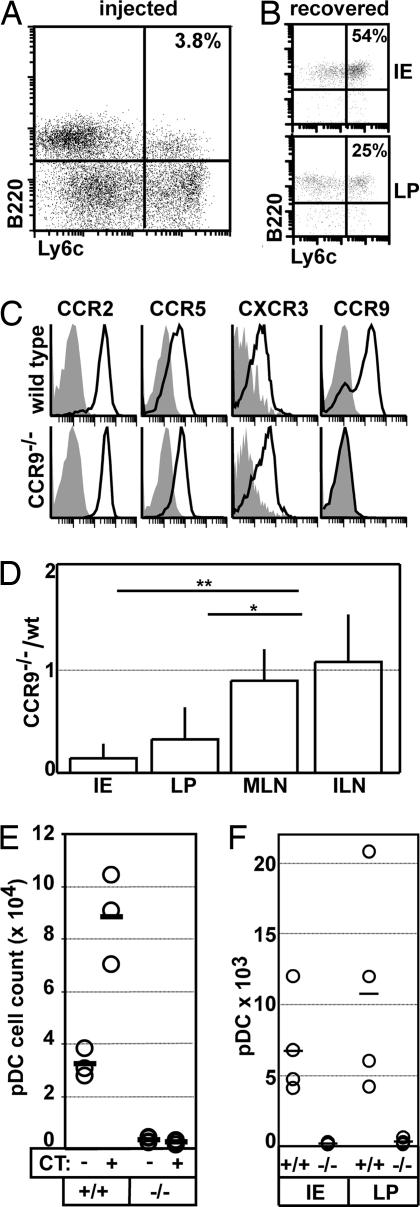
Fig. 4.
CCR9-dependent homing of pDC to the small intestine. (A–D and F) DC were expanded in vivo by treating B6 and CCR9-deficient mice with Flt3L-secreting tumor cells. (A) Splenocytes of B6 donors were analyzed for the presence of pDC (B220+Ly6C+). (B) Nonpurified WT Flt3L-expanded splenocytes were labeled with CFSE and i.v. injected into recipients. The occurrence of donor pDC in the recipient's IE (Upper) and LP (Lower) preparation was analyzed 18 h later (gate on CFSE+ cells). (A and B) Data shown are representative for five animals of two independent experiments. (C) CD11+B220+Ly6C+ pDC in Flt3L-expanded splenocytes of B6 (Upper) and CCR9-deficient mice (Lower) were stained for the expression of different chemokine receptors as indicated. (D) Splenocytes isolated from Flt3L-treated B6 or CCR9-deficient mice were labeled with CFSE and TAMRA, respectively. Cells were adjusted to equal numbers of pDC and injected at a ratio of 1:1 in B6 recipients. After 18 h, recipients were killed, and the ratio of donor B6 and CCR9-deficient pDC was analyzed in the recipient's IE and LP preparation of the intestine and the inguinal (ILN) and mesenteric (MLN) lymph node (mean + SD; n = 5–9 recipients). (E) WT (+/+) and CCR9-deficient (−/−) mice received 10 μg of CT or saline orally. After 1 h, animals were killed, and the number of pDC present in the small intestine IE preparation was determined. Circles represent individual mice; bars are mean values. Similar results were obtained in two additional experiments. (F) CFSE-labeled splenocytes isolated from Flt3L-treated B6 and TAMRA-labeled splenocytes isolated from Flt3L-treated CCR9-deficient mice were adoptively transferred to CCR9-deficient mice at a ratio of 1:1. After 18 h, recipients were gavaged orally with 10 μg of CT. One hour later, mice were killed, and the number of labeled WT (+/+) and CCR9−/− (−/−) pDC isolated from the IE and LP preparation was analyzed. Circles represent individual mice; bars are mean values.