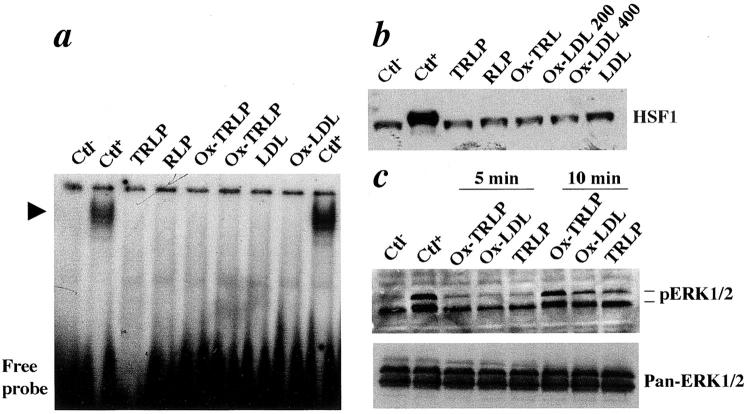
Figure 4.
TRLP, remnant lipoprotein, oxidized TRLP, LDL, and oxidized LDL do not activate HSF-DNA binding. a: Gel mobility shift assay for protein extracts from cultured SMCs. Rat SMCs were treated with TRLP, remnant lipoprotein, oxidized TRLP (100 μg/ml), LDL, and oxidized LDL (100 μg/ml) at 37°C for 1 hour. The cells were washed and harvested with cold Tris-buffered saline. Nuclear proteins were prepared as described in Materials and Methods and protein concentration was determined with a Bio-Rad assay. Protein extracts (5 μg per lane) were incubated with a radiolabeled HSE oligonucleotide on ice for 20 minutes. The gel mobility shift assay was performed in 4% gel. Arrowhead indicates specific HSE-binding complexes. b: Western blot analysis. SMCs were treated with TRLP, remnant lipoprotein, oxidized TRLP, LDL, and oxidized LDL (μg/ml) at 37°C for 12 hours. Protein extracts (50 μg/lane) were separated on 10% sodium dodecyl sulfate-polyacrylamide gel, transferred onto a membrane, and probed with the antibody against HSF1. Immunocomplexes were visualized by a Western blot detection kit. c: Western blot analysis for ERK1/2. Protein extracts from cultured SMCs treated with lipoproteins were separated on 15% sodium dodecyl sulfate-polyacrylamide gel, transferred onto a membrane, and probed with the antibody against phospho-ERK1/2 and pan-ERK1/2. Blots were stripped between antibody incubations. Immunocomplexes were visualized by a Western blot detection kit. Ctl−, negative control without treatment; CtL+, SMCs treated at 42°C for 30 minutes.