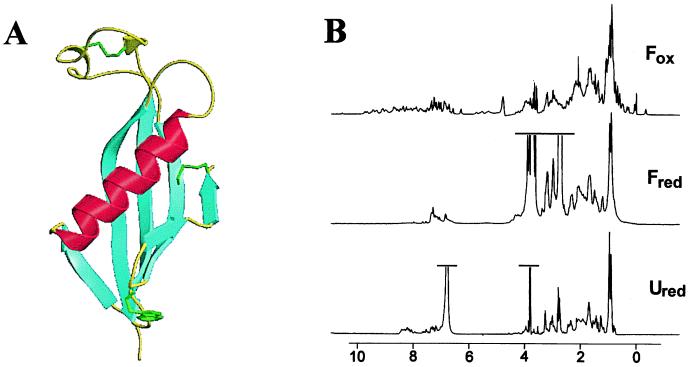
Figure 1.
(A) The three-dimensional structure of chicken cystatin (8–10). The single tryptophan residue is highlighted in green and the disulfide bonds in yellow. (B) One-dimensional 1H-NMR spectra of the different states of cystatin were acquired at 298 K on a Bruker DRX-500 spectrometer and were processed by using Felix (Molecular Simulations, Waltham, MA). Fox conditions were 200 μM protein in 50 mM sodium phosphate buffer (pH 7.0) and 10% D2O. Fred was made by refolding a 1.5 mM denatured sample (5.6 M GdnHCl/50 mM Tris⋅HCl, pH 7.0/20 mM DTT/2 mM EDTA) by diluting 5-fold into 50 mM Tris⋅HCl (pH 7.0) and 20 mM DTT in D2O. Ured conditions were 1.5 mM protein in 50 mM Tris⋅HCl (pH 7.0), 5.6 M GdnHCl, 20 mM DTT, 2 mM EDTA, and 10% D2O.