Figure 4.
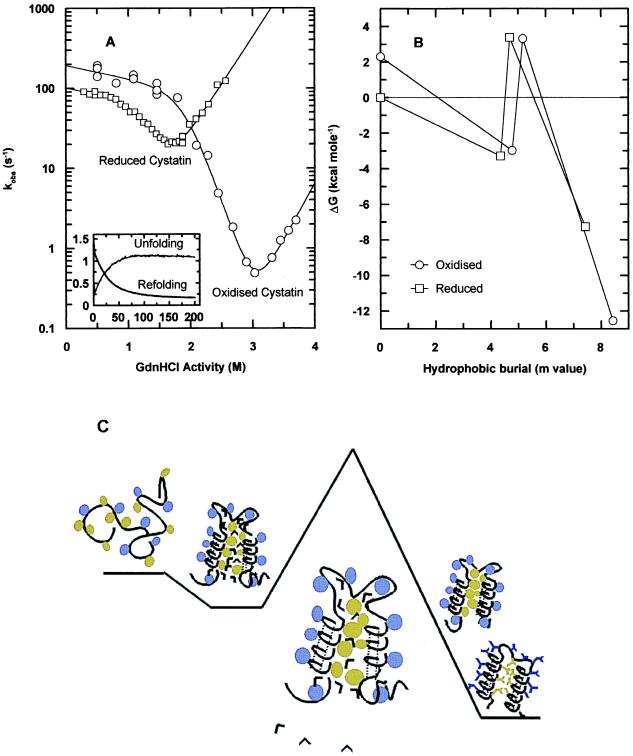
(A) Chevron plots for the folding reactions of Fox and Fred indicating the population of a kinetic intermediate. Open circles represent oxidized cystatin: folding was initiated by 1 + 10 dilution of 11 μM Uox [in 50 mM Tris⋅HCl (pH7.0) and 6.3 M GdnHCl], against 10 volumes of buffer or buffered GdnHCl in an Applied Photophysics (Surrey, U.K.) stopped-flow apparatus (λexcitation = 290 nm and slit width 4 nm). Fluorescence emission above 320 nm was recorded. Unfolding was initiated by 1 + 10 dilution of 11 μM Fox [in 50 mM Tris⋅HCl (pH 7.0)] as above. Open squares represent Fred: Refolding was carried out in two successive steps: a 50 μM stock solution of Ured [in 50 mM Tris⋅HCl (pH7.0) buffer, 6.3 M GdnHCl] was diluted to 3.3 M GdnHCl, where Fred (10 μM) is still not populated significantly (A). This solution was then refolded as for Fox. Unfolding was also carried out in two steps. A 100 μM stock protein solution in 5.6 M GdnHCl was diluted 20-fold, with 50 mM Tris⋅HCl (pH 7.0), before unfolding as for Fox. Fresh Fred was made every 20 min. All solutions contained 20 mM DTT. All experiments were carried out at 20°C. The data were fitted to Eq. 3, and the parameters determined are listed in Table 1. The inset to A shows typical unfolding and refolding transients. (B) A free energy plot for the folding reactions of both Fox (○) and Fred (□) is shown in which the reaction coordinate is the m values of the different states relative to the unfolded state. The plot representing folding in oxidizing conditions is offset vertically with respect to that in a reducing environment to account for the destabilizing effect of the disulfide bonds on Uox. Using the free energies of folding of cystatin in the reduced and oxidized states, and the redox potential measured in the folded state (−5.5 kcal⋅mol−1; results not shown), the resulting thermodynamic cycle provides a value of + 2.3 kcal⋅mol−1 for the free energy of Uox with respect to Ured. In agreement with this, a destabilization of +2.1 kcal⋅mol−1 was obtained from a theoretical calculation of the entropic effect of introducing a cross-link in a random coil polymer (32). (C) A schematic representation of the nature of the states identifiable in the refolding profile is shown. Hydrophilic side chains are shaded in purple, and hydrophobic side chains in green. When conditions favor folding, the protein initially buries many of its hydrophobic residues and forms secondary structure. Such species appear to be significantly native-like in fold topology (28). To bury further hydrophobic side chains, the protein has to expel many of the remaining water molecules, and can only do this at a high enthalpic cost through a transition state that contains structurally constrained water molecules as described (36). Moreover, once past the transition state, rigid packing interactions are only formed when the entropic cost for this immobilization is adequately compensated.