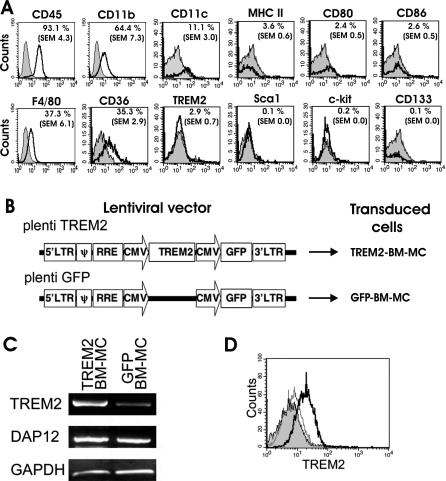
Figure 2. Characterization and TREM2 Transduction of BM-MC.
(A) Flow cytometry analyses of BM-MC. Bone marrow cells were cultured for 10 d in GM-CSF–containing medium and then analyzed by flow cytometry with specific antibodies (open tracings) directed against CD45, CD11b, CD11c, MHC class II, CD80, CD86, F4/80, CD36, TREM2, Sca1, c-kit, and CD133. Cells showed expression of CD45, F4/80, CD11b, CD11c, and CD36. The percentage of positive cells is indicated in the upper-right corner of each histogram. Isotype controls are shown in grey-filled tracings.
(B) Lentiviral vector design. To express TREM2 in BM-MC, the mouse TREM2 coding sequence was cloned under the CMV promoter followed by a second CMV promoter and GFP (plenti TREM2). The same vector without TREM2 was used as a control (plenti GFP).
(C) Gene transcripts in myeloid cells transduced with TREM2 vector (TREM2-BM-MC) or control GFP vector (GFP-BM-MC) for TREM2, DAP12, and the housekeeping gene GAPDH were analyzed by RT-PCR. Gene transcripts for TREM2 were strongly detected in TREM2-BM-MC and weakly in GFP-BM-MC. Both TREM2-transduced myeloid cells and control GFP vector–transduced myeloid cells showed gene transcription for DAP12, the TREM2 adapter and signaling molecule.
(D) Flow cytometry analysis of TREM2-transduced BM-MC (bold line) and GFP-transduced BM-MC (narrow line), both labeled with TREM2-specific antibodies. Isotype control antibody is shown in filled grey. Cell surface expression of TREM2 was detected on myeloid cells after lentiviral transduction with TREM2, but not on control GFP vector–transduced myeloid cells.