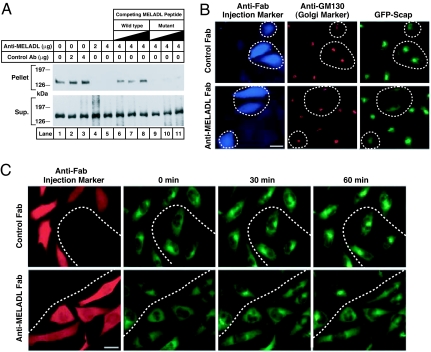
Fig. 2.
Anti-MELADL inhibits Scap transport from ER to Golgi. (A) Binding of COPII proteins to Scap in vitro. On day 0, Scap-deficient SRD-13A cells were set up in 100-mm dishes. On day 2, cells were transfected with 2 μg of pTK-SREBP-2, 0.4 μg of pCMV–Scap, and 0.2 μg of pCMV-Insig-1-Myc. Twelve hours later, cells were switched to sterol-depleting medium containing 1% HPCD and incubated for 1 h at 37°C. Cells were then switched to the same medium without HPCD. After 3 h at 37°C, cells were harvested, and 150 μg of microsomal membranes (16,000 × g pellet) were incubated, in a final volume of 0.3 ml Buffer B, with the indicated amount of affinity-purified control anti-T7 tag or anti-MELADL antibody in absence or presence of increasing amounts (0.2, 0.5, and 1.0 mg) of a 16-aa synthetic peptide corresponding to residues 446–461 of Scap and containing wild-type (lanes 6–8) or a mutant MELADL sequence substituted with AAAAAA (lanes 9–11). After a 30-min incubation on ice, we added 10 μg of a recombinant mutant of GST-Sar-1(H79G; GTPase-defective) and 10 μg of recombinant Flag-Sec23/24 in the presence of 0.5 mM sodium GTP. The Scap/COPII complex was precipitated with anti-FLAG. The resulting supernatant (Sup.) and pellet (5% of Sup.) fractions were subjected to 8% SDS/PAGE and immunoblot analysis with IgG-R139 (anti-Scap). (B) Immunofluorescence. On day 0, CHO/pGFP-Scap cells were set up on 12-mm coverslips as described in Materials and Methods. On day 2, cells were switched to microinjection medium supplemented with 5% FCS, after which Fab fragments (0.2 mg/ml) of either anti-MELADL or control antibody (IgG-R139) were microinjected into the cytoplasm. After 1-h incubation at 37°C, cells were switched to sterol-depleting medium containing 1% HPCD without sterols and incubated for 1 h at 37°C. Cells were then fixed for 15 min in 3.7% formaldehyde in PBS at room temperature and permeabilized for 10 min in methanol at −20°C. Cells were stained with monoclonal IgG-GM130 (1.2 μg/ml, anti-GM130) and visualized with Alexa Fluor 594 goat anti-mouse IgG (1:300 dilution, GM130), and Alexa Fluor 350 goat anti-rabbit IgG (1:200 dilution, microinjected Fab fragments). GFP-Scap was visualized directly. (Scale bar, 25 μm.) (C) Time-lapse imaging. On day 0, CHO/pGFP-Scap cells were set up on 18-mm coverslips as described in Materials and Methods. On day 1, cells were switched to medium A with 5% LPDS and 1% HPCD in the presence of sterols (10 μg/ml 25-HC and 10 μg/ml cholesterol in 0.2% ethanol) and incubated for 16 h. On day 2, cells were refed with microinjection medium with 5% LPDS and 1% HPCD in the presence of sterols, after which Fab fragments of either anti-MELADL or control antibody (IgG-R139) were microinjected into the cytoplasm (34). After 30 min (zero time), the coverslip was mounted into a square holder (Ludin chamber) and positioned on the microscope stage, after which the cells were switched to sterol-depleting imaging medium, and images were collected every 5 min for 75 min. Note that only images taken at 0, 30, and 60 min are presented. During the experiment, cells were kept at 37°C on a microscope enclosed in an environmental chamber. After imaging, the coverslip chamber was removed from the stage after which the cells were fixed in the chamber, permeabilized as described above, and stained with Alexa Fluor 594 conjugated goat anti-rabbit IgG (1:200 dilution). To identify injected cells, the coverslip chamber was mounted back on the microscope stage, which was repositioned to the stored x–y coordinates on the stage. This allowed us to image the same field of cells as detected during live cell imaging. (Scale bar, 25 μm.)