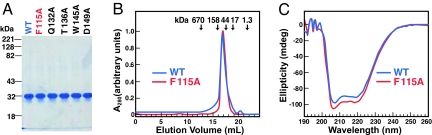
Fig. 1.
Characterization of purified His10–Insig-2–FLAG. (A) Coomassie staining. Recombinant Insig-2 proteins were purified in two steps as described in Materials and Methods. Five micrograms of wild-type His10–Insig-2–FLAG or the indicated mutant version were subjected to 10% SDS/PAGE, and the proteins were visualized with Coomassie brilliant blue R-250 stain (Bio-Rad). Molecular masses of protein standards are indicated. (B) Gel filtration chromatography of purified proteins. Buffer A (100 μl) containing 6 nmol of either His10–Insig-2–FLAG or His10–Insig-2–FLAG(F115A) was loaded onto a Tricorn 10/300 Superose 6 column (Amersham) and chromatographed at a flow rate of 0.4 ml/min. Absorbance at 280 nm was monitored continuously to identify His10–Insig-2–FLAG (blue) or His10-Insig-2(F115A)-FLAG (red). Standard molecular mass markers (thyroglobulin, Mr 670,000; γ globulin, Mr 158,000; ovalbumin, Mr 44,000; myoglobin, Mr 17,000; and vitamin B12, Mr 1350) were chromatographed on the same column (arrows). The apparent molecular mass of His10–Insig-2–FLAG is 80 kDa. (C) Circular dichroism spectroscopy. Circular dichroism of 3 μM His10–Insig-2–FLAG or His10–Insig-2–FLAG(F115A) in buffer A was measured on an Aviv 62DS spectrometer using a 2-mm path length cuvette. The average of 10 spectra is shown.