Figure 4.
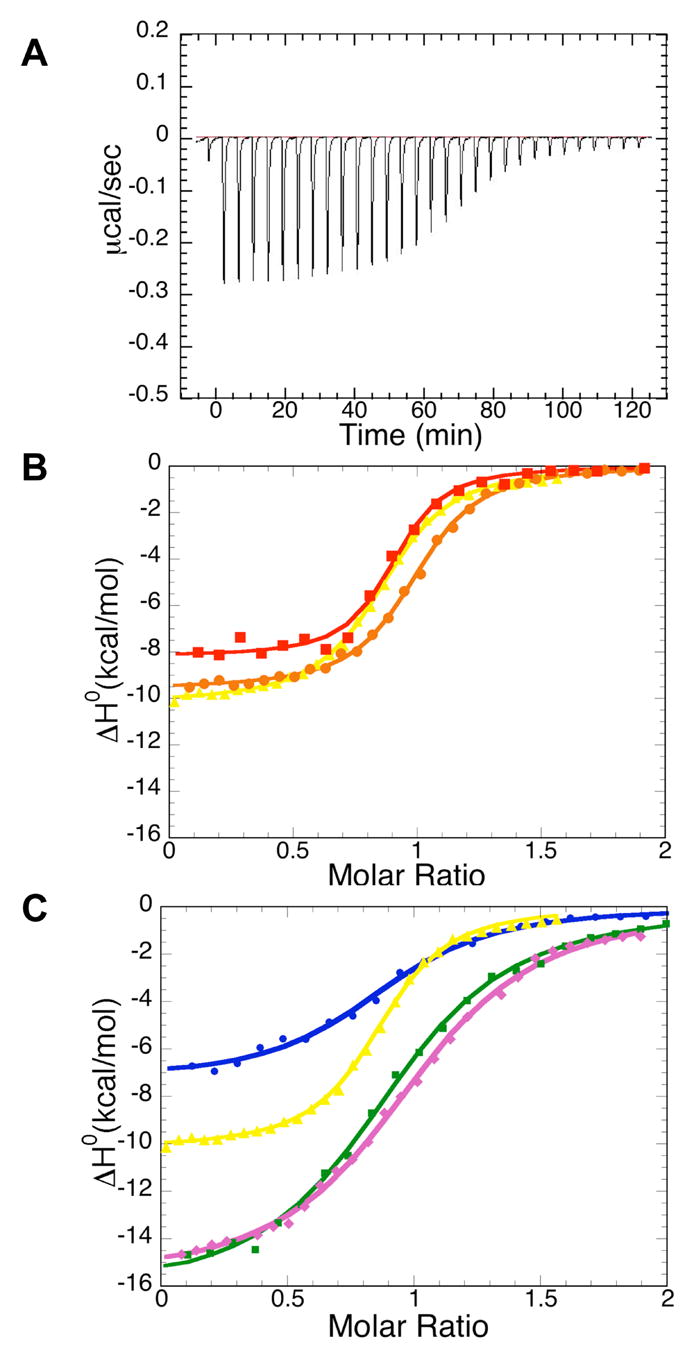
Isothermal calorimetric titrations for binding of the BTD161–349 domain to Notch1 polypeptides. (A) Raw heat signal of an isothermal titration of BTD161–349 with the RAM domain of Notch. The power output is shown as a function of time (seconds). 10μ l increments of 0.12mM RAM were injected into a 1.35 ml cell containing 10μ M BTD161–349. (B) Integrated data for titrations of RAMANKPPD (red filled squares), RAMANK (brown filled circles) and RAM (yellow filled triangles) with the BTD161–349. (C) Integrated data for titrations of RAM (yellow filled triangles), N1RAM13 peptide (purple filled diamonds), N1RAM16 (blue filled circles) and N1RAM22 (green filled squires) with BTD161–349. Solid lines represent nonlinear least-squares fits to the data. All titrations shown here were performed at 30 ° C in 20mM HEPES pH7.5, 100mM NaCl, 1mM EDTA and 2mM BME.
