Figure 5.
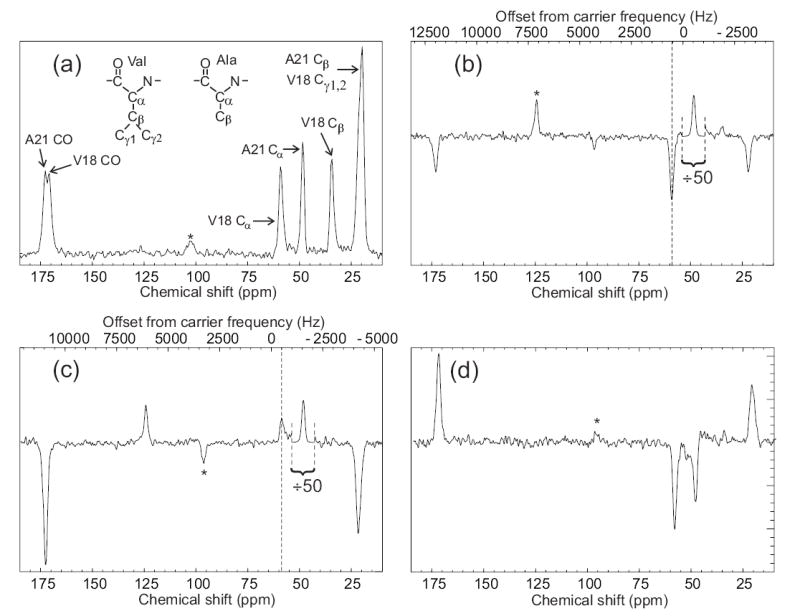
Observation of an intermolecular polarization transfer between Aβ14–23 peptide molecules within amyloid fibrils using SEASHORE. Aβ14–23 molecules were uniformly 15N,13C-labeled at Val18 and Ala21. (a) Conventional 13C NMR spectrum with peak assignments, obtained at 100.4 MHz 13C NMR frequency in 16 scans with 7.00 kHz MAS. (b) Spectrum obtained with the pulse sequence in Fig. 1, with selective excitation of the Ala21 Cα peak, N = 32, m = 2, n = 1, and 7.626 kHz MAS. The rf carrier frequency was set to the midpoint of the Ala21 Cα and Val18 Cα NMR frequencies. This spectrum is the result of 50,000 scans. The Ala21 Cα peak is scaled down by a factor of 50 relative to the rest of the spectrum. Vertical dashed line marks the Val18 Cα NMR frequency. (c) Spectrum obtained as in part b, but with a 1000 Hz shift of the carrier frequency during the SEASHORE period to prevent Ala21-Val18 recoupling. (d) The difference of spectra in parts b and c. Asterisks indicate MAS sideband lines.
