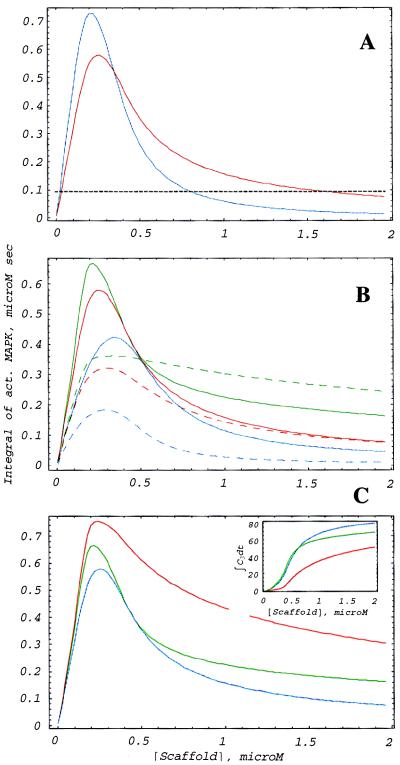
Figure 4.
The scaffold membership and cooperativity of MAPKK and MAPK binding to scaffold affect MAPK activation. (A) Comparison of the effects of two- (red) and three- (blue) member scaffolds on the signaling properties. Assumptions of the three-member scaffold model are the same as the two-member model considered above with the additional assumption of MAPKKK binding to the scaffold. The dashed line illustrates that it takes considerably more two-member than three-member scaffolds to inhibit activity to the same level at high scaffold concentrations. (B) Cooperativity does not affect the property of existence of an optimal scaffold concentration but results in a shift of the optimum. No cooperativity (red), partial positive (green), and partial negative (blue) cooperativity are shown. In partial cooperativity, only inactive kinases interact. The dashed lines of the corresponding color represent C6 normalized as in Fig. 3. (C) Full (red) and partial (green) positive cooperation is compared with noncooperative binding (blue). Inset illustrates the concomitant dependence of C3 (see Fig. 1). In all figures, cooperativity is defined as an increase or decrease in the second kinase association constant by the factor of 10 when the first kinase is bound. See more information in the text.