Abstract
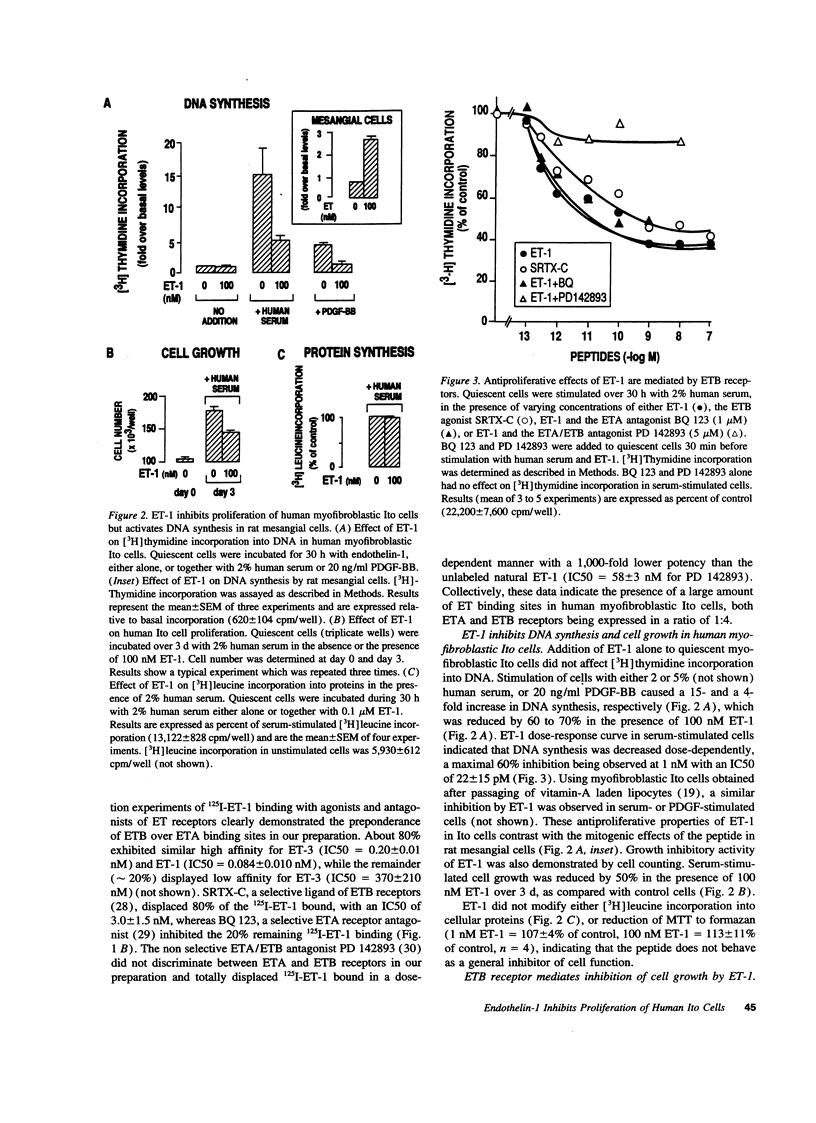
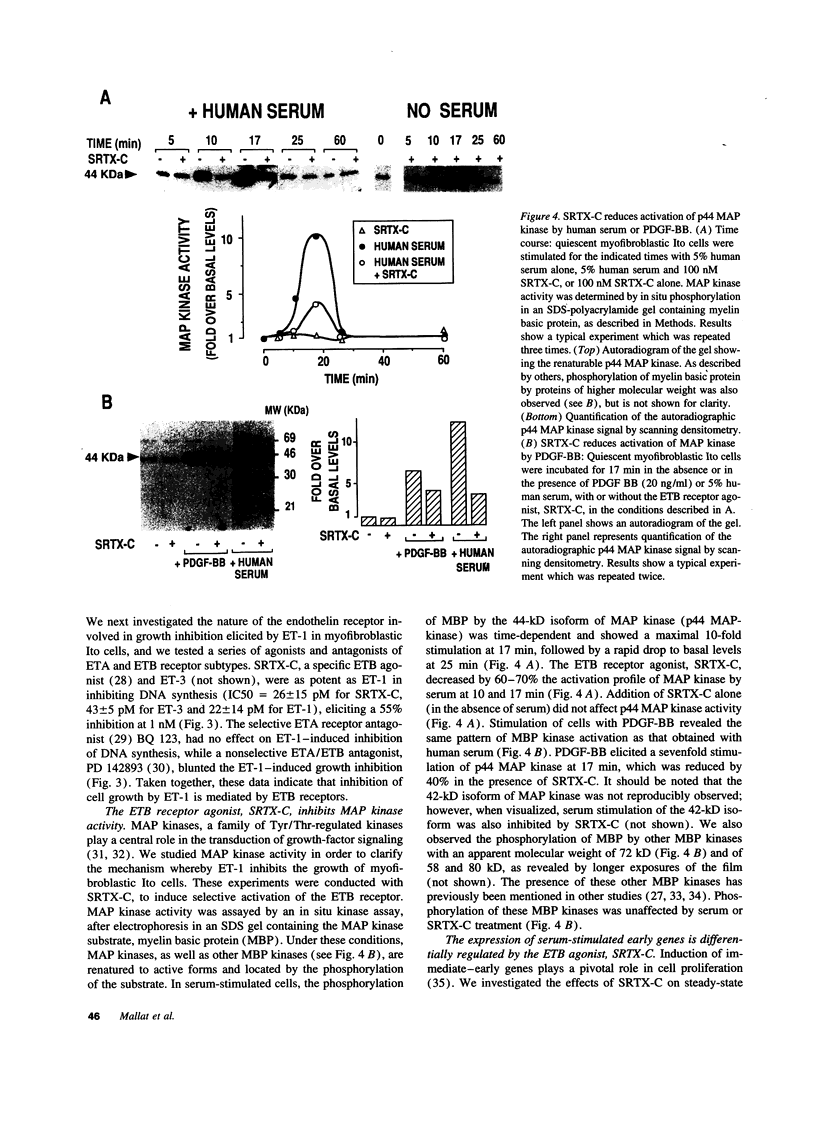
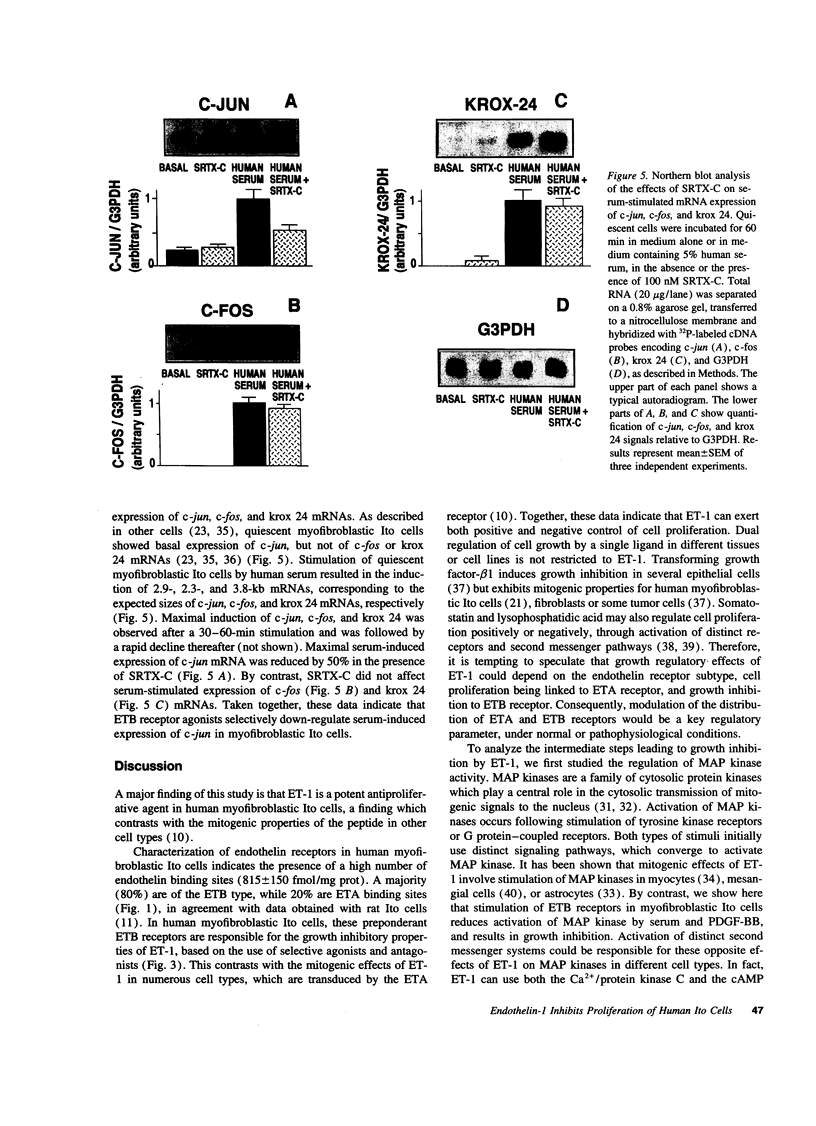
Ito cells play a pivotal role in the development of liver fibrosis associated with chronic liver diseases. During this process, Ito cells acquire myofibroblastic features, proliferate, and synthesize fibrosis components. Considering the reported mitogenic properties of endothelin-1 (ET-1), we investigated its effects on the proliferation of human Ito cells in their myofibroblastic phenotype. Both ET receptor A (ETA: 20%) and ET receptor B (ETB: 80%) binding sites were identified, using a selective ETA antagonist, BQ 123, and a selective ETB agonist, sarafotoxin S6C (SRTX-C). ET-1 did not stimulate proliferation of myofibroblastic Ito cells. In contrast, ET-1 inhibited by 60% DNA synthesis and proliferation of cells stimulated with either human serum or platelet-derived growth factor -BB (PDGF-BB). PD 142893, a nonselective ETA/ETB antagonist totally blunted this effect. SRTX-C was as potent as ET-1, while BQ 123 did not affect ET-1-induced growth inhibition. Analysis of the intermediate steps leading to growth-inhibition by ET-1 revealed that activation of mitogen-activated protein kinase by serum or PDGF-BB was decreased by 50% in the presence of SRTX-C. In serum-stimulated cells, SRTX-C reduced c-jun mRNA expression by 50% whereas c-fos or krox 24 mRNA expression were not affected. We conclude that ET-1 binding to ETB receptors causes a potent growth inhibition of human myofibroblastic Ito cells, which suggests that this peptide could play a key role in the negative control of liver fibrogenesis. Our results also point out that, in addition to its well known promitogenic effects, ET-1 may also exert negative control of growth on specific cells.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Battistini B., Chailler P., D'Orléans-Juste P., Brière N., Sirois P. Growth regulatory properties of endothelins. Peptides. 1993 Mar-Apr;14(2):385–399. doi: 10.1016/0196-9781(93)90057-n. [DOI] [PubMed] [Google Scholar]
- Bluhm R. E., Frazer M. G., Vore M., Pinson C. W., Badr K. F. Endothelins 1 and 3: potent cholestatic agents secreted and excreted by the liver that interact with cyclosporine. Hepatology. 1993 Oct;18(4):961–968. doi: 10.1002/hep.1840180430. [DOI] [PubMed] [Google Scholar]
- Blumer K. J., Johnson G. L. Diversity in function and regulation of MAP kinase pathways. Trends Biochem Sci. 1994 Jun;19(6):236–240. doi: 10.1016/0968-0004(94)90147-3. [DOI] [PubMed] [Google Scholar]
- Bogoyevitch M. A., Glennon P. E., Andersson M. B., Clerk A., Lazou A., Marshall C. J., Parker P. J., Sugden P. H. Endothelin-1 and fibroblast growth factors stimulate the mitogen-activated protein kinase signaling cascade in cardiac myocytes. The potential role of the cascade in the integration of two signaling pathways leading to myocyte hypertrophy. J Biol Chem. 1994 Jan 14;269(2):1110–1119. [PubMed] [Google Scholar]
- Cazaubon S., Parker P. J., Strosberg A. D., Couraud P. O. Endothelins stimulate tyrosine phosphorylation and activity of p42/mitogen-activated protein kinase in astrocytes. Biochem J. 1993 Jul 15;293(Pt 2):381–386. doi: 10.1042/bj2930381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao T. S., Byron K. L., Lee K. M., Villereal M., Rosner M. R. Activation of MAP kinases by calcium-dependent and calcium-independent pathways. Stimulation by thapsigargin and epidermal growth factor. J Biol Chem. 1992 Oct 5;267(28):19876–19883. [PubMed] [Google Scholar]
- Chen J., Iyengar R. Suppression of Ras-induced transformation of NIH 3T3 cells by activated G alpha s. Science. 1994 Mar 4;263(5151):1278–1281. doi: 10.1126/science.8122111. [DOI] [PubMed] [Google Scholar]
- Chiu R., Angel P., Karin M. Jun-B differs in its biological properties from, and is a negative regulator of, c-Jun. Cell. 1989 Dec 22;59(6):979–986. doi: 10.1016/0092-8674(89)90754-x. [DOI] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Davis R. J. The mitogen-activated protein kinase signal transduction pathway. J Biol Chem. 1993 Jul 15;268(20):14553–14556. [PubMed] [Google Scholar]
- Denizot F., Lang R. Rapid colorimetric assay for cell growth and survival. Modifications to the tetrazolium dye procedure giving improved sensitivity and reliability. J Immunol Methods. 1986 May 22;89(2):271–277. doi: 10.1016/0022-1759(86)90368-6. [DOI] [PubMed] [Google Scholar]
- Fausto N. Multifunctional roles for transforming growth factor-beta 1. Lab Invest. 1991 Nov;65(5):497–499. [PubMed] [Google Scholar]
- Friedman S. L. Seminars in medicine of the Beth Israel Hospital, Boston. The cellular basis of hepatic fibrosis. Mechanisms and treatment strategies. N Engl J Med. 1993 Jun 24;328(25):1828–1835. doi: 10.1056/NEJM199306243282508. [DOI] [PubMed] [Google Scholar]
- Gandhi C. R., Stephenson K., Olson M. S. A comparative study of endothelin- and platelet-activating-factor-mediated signal transduction and prostaglandin synthesis in rat Kupffer cells. Biochem J. 1992 Jan 15;281(Pt 2):485–492. doi: 10.1042/bj2810485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandhi C. R., Stephenson K., Olson M. S. Endothelin, a potent peptide agonist in the liver. J Biol Chem. 1990 Oct 15;265(29):17432–17435. [PubMed] [Google Scholar]
- Goto M., Takei Y., Kawano S., Nagano K., Tsuji S., Masuda E., Nishimura Y., Okumura S., Kashiwagi T., Fusamoto H. Endothelin-1 is involved in the pathogenesis of ischemia/reperfusion liver injury by hepatic microcirculatory disturbances. Hepatology. 1994 Mar;19(3):675–681. doi: 10.1002/hep.1840190319. [DOI] [PubMed] [Google Scholar]
- Hendriks H. F., Brouwer A., Knook D. L. The role of hepatic fat-storing (stellate) cells in retinoid metabolism. Hepatology. 1987 Nov-Dec;7(6):1368–1371. doi: 10.1002/hep.1840070630. [DOI] [PubMed] [Google Scholar]
- Housset C., Rockey D. C., Bissell D. M. Endothelin receptors in rat liver: lipocytes as a contractile target for endothelin 1. Proc Natl Acad Sci U S A. 1993 Oct 15;90(20):9266–9270. doi: 10.1073/pnas.90.20.9266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu R. M., Levin E. R. Astrocyte growth is regulated by neuropeptides through Tis 8 and basic fibroblast growth factor. J Clin Invest. 1994 Apr;93(4):1820–1827. doi: 10.1172/JCI117167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jouneaux C., Mallat A., Serradeil-Le Gal C., Goldsmith P., Hanoune J., Lotersztajn S. Coupling of endothelin B receptors to the calcium pump and phospholipase C via Gs and Gq in rat liver. J Biol Chem. 1994 Jan 21;269(3):1845–1851. [PubMed] [Google Scholar]
- Karin M., Smeal T. Control of transcription factors by signal transduction pathways: the beginning of the end. Trends Biochem Sci. 1992 Oct;17(10):418–422. doi: 10.1016/0968-0004(92)90012-x. [DOI] [PubMed] [Google Scholar]
- Karne S., Jayawickreme C. K., Lerner M. R. Cloning and characterization of an endothelin-3 specific receptor (ETC receptor) from Xenopus laevis dermal melanophores. J Biol Chem. 1993 Sep 5;268(25):19126–19133. [PubMed] [Google Scholar]
- Kawada N., Tran-Thi T. A., Klein H., Decker K. The contraction of hepatic stellate (Ito) cells stimulated with vasoactive substances. Possible involvement of endothelin 1 and nitric oxide in the regulation of the sinusoidal tonus. Eur J Biochem. 1993 Apr 15;213(2):815–823. doi: 10.1111/j.1432-1033.1993.tb17824.x. [DOI] [PubMed] [Google Scholar]
- Lissoos T. W., Beno D. W., Davis B. H. 1,25-Dihydroxyvitamin D3 activates Raf kinase and Raf perinuclear translocation via a protein kinase C-dependent pathway. J Biol Chem. 1993 Nov 25;268(33):25132–25138. [PubMed] [Google Scholar]
- Marx J. Two major signal pathways linked. Science. 1993 Nov 12;262(5136):988–990. doi: 10.1126/science.8257559. [DOI] [PubMed] [Google Scholar]
- Masaki T., Yanagisawa M., Goto K. Physiology and pharmacology of endothelins. Med Res Rev. 1992 Jul;12(4):391–421. doi: 10.1002/med.2610120405. [DOI] [PubMed] [Google Scholar]
- Miller R. C., Pelton J. T., Huggins J. P. Endothelins--from receptors to medicine. Trends Pharmacol Sci. 1993 Feb;14(2):54–60. doi: 10.1016/0165-6147(93)90031-e. [DOI] [PubMed] [Google Scholar]
- Nakamichi K., Ihara M., Kobayashi M., Saeki T., Ishikawa K., Yano M. Different distribution of endothelin receptor subtypes in pulmonary tissues revealed by the novel selective ligands BQ-123 and [Ala1,3,11,15]ET-1. Biochem Biophys Res Commun. 1992 Jan 15;182(1):144–150. doi: 10.1016/s0006-291x(05)80123-8. [DOI] [PubMed] [Google Scholar]
- Nebreda A. R. Inactivation of MAP kinases. Trends Biochem Sci. 1994 Jan;19(1):1–2. doi: 10.1016/0968-0004(94)90163-5. [DOI] [PubMed] [Google Scholar]
- Pagès G., Lenormand P., L'Allemain G., Chambard J. C., Meloche S., Pouysségur J. Mitogen-activated protein kinases p42mapk and p44mapk are required for fibroblast proliferation. Proc Natl Acad Sci U S A. 1993 Sep 15;90(18):8319–8323. doi: 10.1073/pnas.90.18.8319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Castillo A., Pipaón C., García I., Alemany S. NGFI-A gene expression is necessary for T lymphocyte proliferation. J Biol Chem. 1993 Sep 15;268(26):19445–19450. [PubMed] [Google Scholar]
- Peterson G. L. A simplification of the protein assay method of Lowry et al. which is more generally applicable. Anal Biochem. 1977 Dec;83(2):346–356. doi: 10.1016/0003-2697(77)90043-4. [DOI] [PubMed] [Google Scholar]
- Pinzani M., Failli P., Ruocco C., Casini A., Milani S., Baldi E., Giotti A., Gentilini P. Fat-storing cells as liver-specific pericytes. Spatial dynamics of agonist-stimulated intracellular calcium transients. J Clin Invest. 1992 Aug;90(2):642–646. doi: 10.1172/JCI115905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rieder H., Ramadori G., Meyer zum Büschenfelde K. H. Sinusoidal endothelial liver cells in vitro release endothelin--augmentation by transforming growth factor beta and Kupffer cell-conditioned media. Klin Wochenschr. 1991 Jun 18;69(9):387–391. doi: 10.1007/BF01647411. [DOI] [PubMed] [Google Scholar]
- Ruiz-Torres P., Lucio F. J., González-Rubio M., Rodríguez-Puyol M., Rodríguez-Puyol D. A dual effect of somatostatin on the proliferation of cultured rat mesangial cells. Biochem Biophys Res Commun. 1993 Sep 15;195(2):1057–1062. doi: 10.1006/bbrc.1993.2151. [DOI] [PubMed] [Google Scholar]
- Schmitt-Gräff A., Krüger S., Bochard F., Gabbiani G., Denk H. Modulation of alpha smooth muscle actin and desmin expression in perisinusoidal cells of normal and diseased human livers. Am J Pathol. 1991 May;138(5):1233–1242. [PMC free article] [PubMed] [Google Scholar]
- Serradeil-Le Gal C., Jouneaux C., Sanchez-Bueno A., Raufaste D., Roche B., Préaux A. M., Maffrand J. P., Cobbold P. H., Hanoune J., Lotersztajn S. Endothelin action in rat liver. Receptors, free Ca2+ oscillations, and activation of glycogenolysis. J Clin Invest. 1991 Jan;87(1):133–138. doi: 10.1172/JCI114962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonson M. S., Jones J. M., Dunn M. J. Differential regulation of fos and jun gene expression and AP-1 cis-element activity by endothelin isopeptides. Possible implications for mitogenic signaling by endothelin. J Biol Chem. 1992 Apr 25;267(12):8643–8649. [PubMed] [Google Scholar]
- Sokolovsky M. Endothelins and sarafotoxins: physiological regulation, receptor subtypes and transmembrane signaling. Pharmacol Ther. 1992;54(2):129–149. doi: 10.1016/0163-7258(92)90030-4. [DOI] [PubMed] [Google Scholar]
- Stojilković S. S., Catt K. J. Neuroendocrine actions of endothelins. Trends Pharmacol Sci. 1992 Oct;13(10):385–391. doi: 10.1016/0165-6147(92)90118-p. [DOI] [PubMed] [Google Scholar]
- Tigyi G., Dyer D. L., Miledi R. Lysophosphatidic acid possesses dual action in cell proliferation. Proc Natl Acad Sci U S A. 1994 Mar 1;91(5):1908–1912. doi: 10.1073/pnas.91.5.1908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Simonson M. S., Pouysségur J., Dunn M. J. Endothelin rapidly stimulates mitogen-activated protein kinase activity in rat mesangial cells. Biochem J. 1992 Oct 15;287(Pt 2):589–594. doi: 10.1042/bj2870589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams D. L., Jr, Jones K. L., Pettibone D. J., Lis E. V., Clineschmidt B. V. Sarafotoxin S6c: an agonist which distinguishes between endothelin receptor subtypes. Biochem Biophys Res Commun. 1991 Mar 15;175(2):556–561. doi: 10.1016/0006-291x(91)91601-8. [DOI] [PubMed] [Google Scholar]
- Win K. M., Charlotte F., Mallat A., Cherqui D., Martin N., Mavier P., Preaux A. M., Dhumeaux D., Rosenbaum J. Mitogenic effect of transforming growth factor-beta 1 on human Ito cells in culture: evidence for mediation by endogenous platelet-derived growth factor. Hepatology. 1993 Jul;18(1):137–145. [PubMed] [Google Scholar]
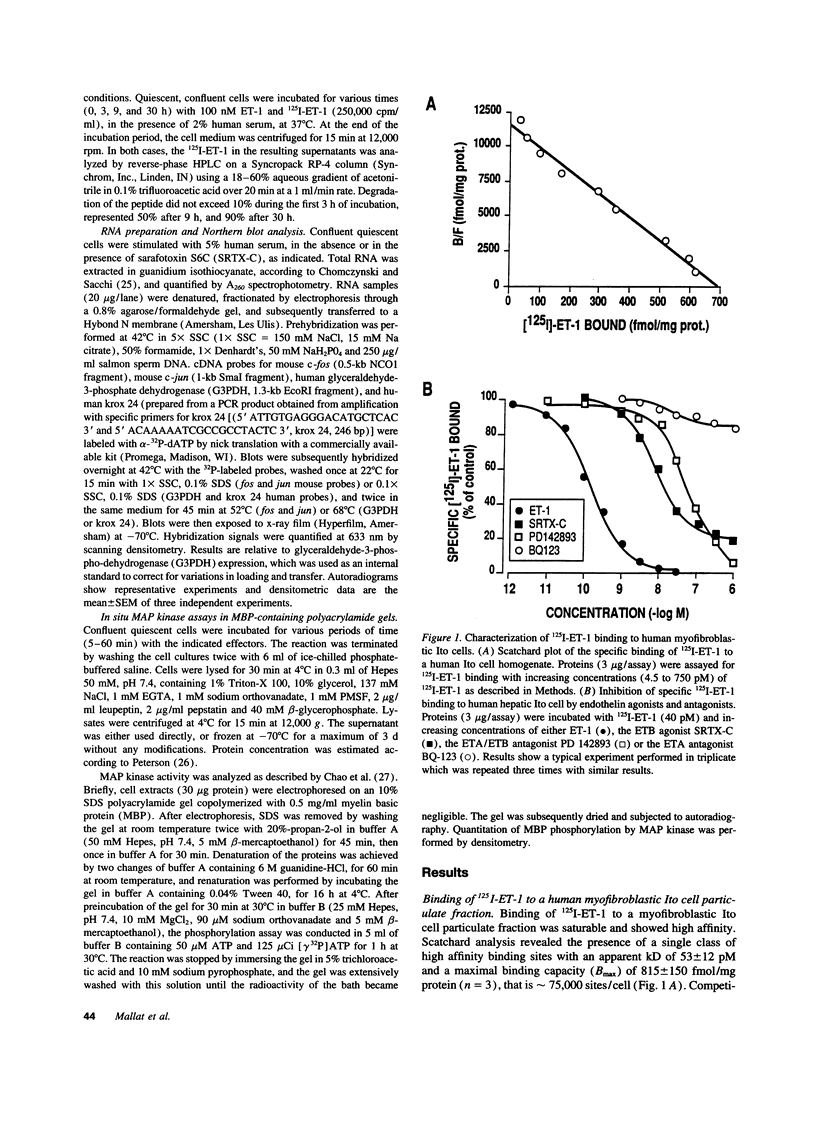
- Yanagisawa M., Kurihara H., Kimura S., Tomobe Y., Kobayashi M., Mitsui Y., Yazaki Y., Goto K., Masaki T. A novel potent vasoconstrictor peptide produced by vascular endothelial cells. Nature. 1988 Mar 31;332(6163):411–415. doi: 10.1038/332411a0. [DOI] [PubMed] [Google Scholar]