Table 1.
Binding and functional data for D2 compounds.
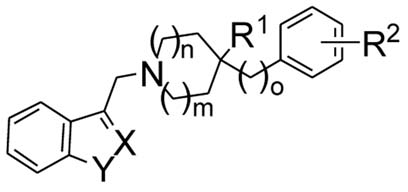 | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Compd. | m | n | o | X-Y | R1 | R2 | D2a | D3a | D4a | D3/D2 | D4/D2 | D2 functionb | D3 functionb |
| Ki [nM] ± SEM | EC50 [nM] ± SEM | ||||||||||||
| 5 | 1 | 1 | 0 | CH-NH | OH | 2′-Cl | 382 ±33 | 1860 ± 340 | 2820 ± 64 | 5 | 7 | 586 ± 210 | ND |
| 6 | 1 | 1 | 0 | CH-NH | OH | 2′,3′-Cl | 26.1 ± 2.0 | 273 ± 42 | 1550 ± 190 | 10 | 59 | 34.3 ± 6.8 | 232 ±7.7 |
| 7 | 1 | 1 | 0 | CH-NH | OH | 3′-Cl | 129 ± 22 | 450 ± 80 | 3540 ± 660 | 3 | 27 | 83.4 ± 10 | ND |
| 8 | 1 | 1 | 0 | CH-NH | OH | 3′,4′-Cl | 6.9 ± 1.0 | 111 ± 17 | 1100 ± 210 | 16 | 159 | 15.3 ± 0.8 | 41.5 ± 9.1 |
| 9 | 1 | 1 | 0 | CH-NH | OH | 3′-CF3 | 106 ± 15 | 570 ± 44 | 3750 ± 140 | 5 | 35 | 69.1 ± 23 | ND |
| 10 | 1 | 1 | 0 | CH-NH | OH | 4′-F | 127 ± 7.2 | 490 ± 74 | 6090 ± 580 | 4 | 48 | ND | ND |
| 11 | 1 | 1 | 0 | CH-NH | OH | 4′-OH | 5360 ± 1100 | 16800 ± 6500 | 1360 ± 270 | 3 | 0.3 | ND | ND |
| 12 | 1 | 1 | 0 | CH-NH | OH | 4′-OMe | 177 ± 61 | 2130 ± 300 | 679 ± 60 | 12 | 4 | ND | ND |
| 13 | 1 | 1 | 0 | CH-NH | OH | 4′-OHex | 159 ± 60 | 1950 ± 350 | 1490 ± 150 | 12 | 9 | 216 ± 1.7 | ND |
| 14 | 1 | 1 | 0 | CH-NH | OH | 4′-Ph | 98.4 ± 19 | 467 ± 38 | 129 ± 26 | 5 | 1 | ND | ND |
| 15 | 2 | 1 | 0 | CH-NH | OH | 4′-Cl | 201 ± 21 | 2100 ± 490 | 3870 ± 770 | 10 | 19 | 260 ± 91 | ND |
| 16 | 1 | 1 | 1 | CH-NH | OH | 4′-Cl | 2890 ± 560 | 5480 ± 2900 | 3930 ± 1200 | 2 | 1 | ND | ND |
| 17 | 2 | 0 | 0 | CH-NH | OH | 4′-Cl | 4265 ± 2280 | 4270 ± 400 | 2440 ± 1400 | 1 | 1 | ND | ND |
| 18 | 1 | 1 | 0 | CH-NH | Me | 4′-Cl | 992 ± 192 | 2100 ± 150 | 2750 ± 140 | 2 | 3 | 314 ± 4.2 | 347±7.4 |
| 19 | 1 | 1 | 0 | CH-NH | NH2 | 4′-Cl | 184 ± 40 | 382 ± 55 | 2293 ± 545 | 2 | 12 | ND | ND |
| 20 | 1 | 1 | 0 | CH-NH | NHAc | 4′-Cl | 83.1 ± 47 | 193 ± 80 | 348 ± 39 | 2 | 4 | 24.8 ± 7.6 | ND |
| 21 | 1 | 1 | 0 | CH-NH | F | 4′-Cl | 120 ± 34 | 211 ± 67 | 365 ± 63 | 2 | 3 | ND | ND |
| 22 | 1 | 1 | 0 | CH-NH | CN | 4′-Cl | 199 ± 24 | 684 ± 180 | 2270 ± 350 | 3 | 11 | 225 ± 80 | ND |
| 23 | 1 | 1 | 0 | CH-NH | COOMe | 4′-Cl | 60.0 ± 9.5 | 544 ± 128 | 3930 ± 2500 | 9 | 66 | 76.7 ± 21 | ND |
| 24 | 1 | 1 | 0 | CH-NH | COOEt | 4′-Cl | 58.8 ± 20 | 724 ± 120 | 3520 ± 1320 | 12 | 60 | ND | ND |
| 25 | 1 | 1 | 0 | CH-NH | CH2NH2 | 4′-Cl | >10000 | >5000 | >20000 | ND | ND | ||
| 26 | 1 | 1 | 0 | CH-NH | CH2NHAc | 4′-Cl | 2000 ± 640 | 12500 ±1300 | 4450 ± 750 | 6 | 2 | ND | ND |
| 27 | 1 | 1 | 0 | CH-NH | CH2OH | 4′-Cl | 2370 ± 560 | 106 ±19 | 1940 ± 250 | 0.04 | 1 | ND | ND |
| 28 | 1 | 1 | 0 | CH-NH | OMe | 4′-Cl | 68.1 ± 22 | 262 ± 73 | 34.1 ± 6.0 | 4 | 1 | 34.7 ± 2.9 | 248 ± 84 |
| 29 | 1 | 1 | 0 | CH-NH | OAllyl | 4′-Cl | 165 ± 91 | 718 ± 35 | 1190 ± 360 | 4 | 7 | ND | ND |
| 30 | 1 | 1 | 0 | CMe-NH | OH | 4′-Cl | 15.4 ± 14 | 44.1 ± 11 | 2990 ± 180 | 3 | 194 | 2.13 ± 0.3 | 20.2 ± 4.2 |
| 31 | 1 | 1 | 0 | N-NH | OH | 3′,4′-Cl | 438 ± 46 | 156 ± 22 | 677 ± 148 | 0.4 | 2 | ND | ND |
| 32 | 1 | 1 | 0 | N-NH | OH | 4′-Cl | 548 ± 80 | 440 ± 88 | 2146 ± 616 | 1 | 4 | ND | ND |
| 33 | 1 | 1 | 0 | CH-O | OH | 3′,4′-Cl | 21.0 ± 4 | 28.0 ± 6 | 167 ± 35 | 1 | 8 | ND | ND |
| 34 | 1 | 1 | 0 | CH-O | OH | 4′-Cl | 21.0 ± 4 | 53.0 ± 12.5 | 404 ± 24 | 3 | 19 | ND | ND |
| 35 | 1 | 1 | 0 | CH-O | OH | 4′-SMe | 54.0 ±13 | 139 ± 7.9 | 153 ± 35 | 3 | 3 | ND | ND |
| 36 | 1 | 1 | 0 | CH-NMe | OH | 4′-Cl | 29.6 ± 6.2 | 238 ± 100 | 5140 ± 3330 | 8 | 174 | 20.0 ± 4.5 | 98.6 ± 29 |
| 37 | 1 | 1 | 0 | CH-NHex | OH | 4′-Cl | 188 ± 27 | 488 ± 280 | 8890 ± 5600 | 3 | 47 | 166.0 ± 35 | 661 ± 56 |
| 38 | 1 | 1 | 0 | n/ac | OH | 4′-Cl | >10000 | ND | >20000 | ND | ND | ||
| 2a11 | 1 | 1 | 0 | CH-NH | OH | 4′-SMe | 23.9 ± 5.5 | 638 ± 159 | 319 ± 58 | 27 | 13 | ND | ND |
| 2b11 | 1 | 1 | 0 | CH-NHd | OH | 4′-SMe | 5.5 ± 0.1 | 580 ± 92 | 567 ± 140 | 105 | 103 | ND | ND |
| 1, L741,62610 | 1 | 1 | 0 | CH-NH | OH | 4′-Cl | 11.2 ± 0.8 | 163 ± 32 | 1520 ± 280 | 15 | 136 | 4.46 ± 0.9 | 90.4 ± 15 |
Inhibtion of binding assay in HEK 293 cells transfected with either hD2L, hD3, or hD4 dopamine receptors, radioligand 125I-IABN.15, 16
Functional assays using inhibition of quinpirole stimulation in hD2 and hD3 receptors transfected into CHO cells. These data were obtained through the service of CTDP, Division of Treatment Research and Development, NIDA, using a contract (N01DA-1-8816) protocol. ND = not determined.
1-((1H-indol-2-yl)methyl)-4-(4′-chlorophenyl)piperidin-4-ol.
1-((5-Methoxy-1H-indol-3-yl)methyl)-4-(4′-(methylthio)phenyl)piperidin-4-ol
