Figure 1.
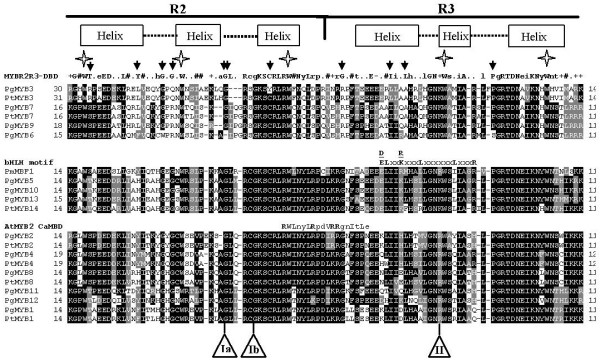
Alignment of predicted MYB domain protein sequences from spruce and pine. Amino acid sequence alignments of the 21 conifer MYB R2R3 domains were obtained with Clustal W (see Methods) and then separated into three groups based on their homologies to the consensus R2R3-MYB DNA-binding domain (MYBR2R3-DBD, top panel), the bHLH protein-binding motif (bHLH motif, middle panel) or the Arabidopsis calmodulin-interaction motif (AtMYB2 CaMBD, bottom panel), as indicated. Black shading indicates identical amino acid residues and grey shading the similar residues that agree with the fraction sequence of 0,4 (BoxShade 3.21) and dashes indicate gaps. The numbers on the left and right indicate the amino acid position relative to the translation start codon. The boxes and dotted line above the sequences show the predicted helix and turn structures in the R2 and R3 regions of the MYB domain. Stars show positions of conserved tryptophan residues and black arrows indicate unusual amino acid residues compared to the consensus amino acid sequence of the MYB DNA-binding domains of several plant R2R3-MYB proteins described by Avila et al. [27]. The bHLH protein-binding motif ([DE]L × 2 [RK] × 3L × 6L × 3R) identified by Zimmerman et al. [28] and the calmodulin-interaction motif [29] are shown above the middle and bottom panels, respectively (major amino acids in upper-case, bold). Ia or Ib and II indicate the positions of the first and second introns, respectively (Ib is specific to PgMYB3). Accession numbers of the newly identified spruce and pine MYBs are listed in Methods. Pg, Picea glauca; Pt, Pinus taeda; Pm, Picea mariana; At, Arabidopsis thaliana.
